FIGURE 1.
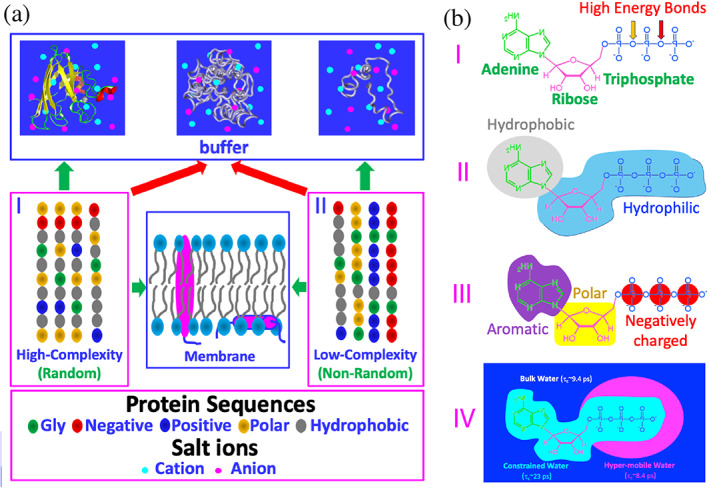
Sequence‐structure relationship of proteins and structure–function relationship of ATP. (a) Based on the sequences as represented by five types of amino acids, proteins can be classified into high‐complexity or random (I), and low‐complexity or non‐random (II) sequences. A portion of proteins of high‐complexity sequence can fold into uniquely‐defined structures soluble in vivo with high concentrations of salts (~150 mM), while many of proteins of low‐complexity sequence remain intrinsically disordered. Interestingly, a large amount of proteins of both high‐ and low‐complexity sequences appear to be aggregation‐prone or even insoluble in vivo. Furthermore, ~30% proteins from both sequences can fold in the membrane environments. (b) ATP has unique structural properties and thus may act as: (I) energy currency by hydrolysis of high energy bonds; (II) biological hydrotrope with the presence of hydrophobic adenine and hydrophilic ribose and triphosphate; (III) bivalent binder by the aromatic purine ring and highly negatively charged triphosphate chain; and (IV) hydration mediator resulting from its unique hydration structure previously derived from the results of microwave dielectric spectroscopy, which was modeled to contain “constrained water” with dielectric relaxation time (τc) of ~23 ps, as well as “hyper‐mobile water” with τc of ~8.4 ps, even smaller than that of bulk water (9.4 ps)
