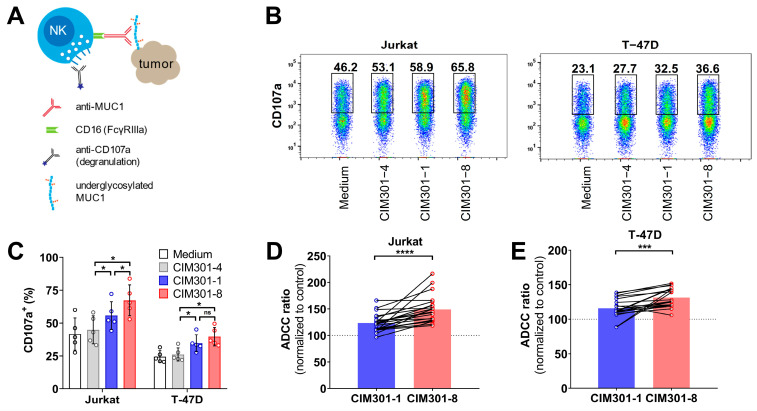
Figure 5.
Defucosylation of humanized anti-MUC1 antibodies further enhances specific anti-tumor responses mediated by primary NK cells. (A) Scheme outlining the analysis of NK cell degranulation and cytotoxicity in co-cultures of primary human NK cells and MUC1 (S)Tn+ tumor cells in the presence of humanized anti-MUC1 antibodies. (B) Flow cytometric analysis of NK cell degranulation after 4 h co-culture in the presence of anti-MUC1 antibodies (regular CIM301-1 and defucosylated CIM301-8) or control antibodies (CIM301-4) at 1 μg/mL. Per condition, one representative sample of CD56+ NK cells is shown, with gates and numbers indicating the fraction of degranulation NK cells. (C) Quantification of the degranulation assays described in (B). Pooled data from five independent experiments performed with different NK cells donors at different time points. Differences between groups were tested using repeated measures one-way ANOVA with Šídák’s multiple comparisons test. (D) Antibody-dependent cell-mediated cytotoxicity (ADCC) assay using human NK cells co-cultured with Jurkat tumor cells with or without 1 µg/mL regular (CIM301-1) or defucosylated (CIM301-8) antibodies. Dots represent individual data points from six independent experiments with four different effectors: target ratios after normalization to the CIM301-4 control antibody. Dotted line indicates the CIM301-4 baseline level (100%). (E) as (D), but using T-47D tumor cells as target cells. Differences in ADCC (panels (D,E)) were calculated using repeated measures one-way ANOVA with Tukey’s multiple comparisons test. p-values for comparisons to the control antibody for both antibodies using both tumor cells lines were <0.0001. p < 0.05 (*); Not significant (n.s.); p < 0.001 (***); p < 0.0001 (****).