Figure 1. A roadmap for reverse-translational pipelines to interrogate the neural circuitry underlying pain affect and opioid analgesia.
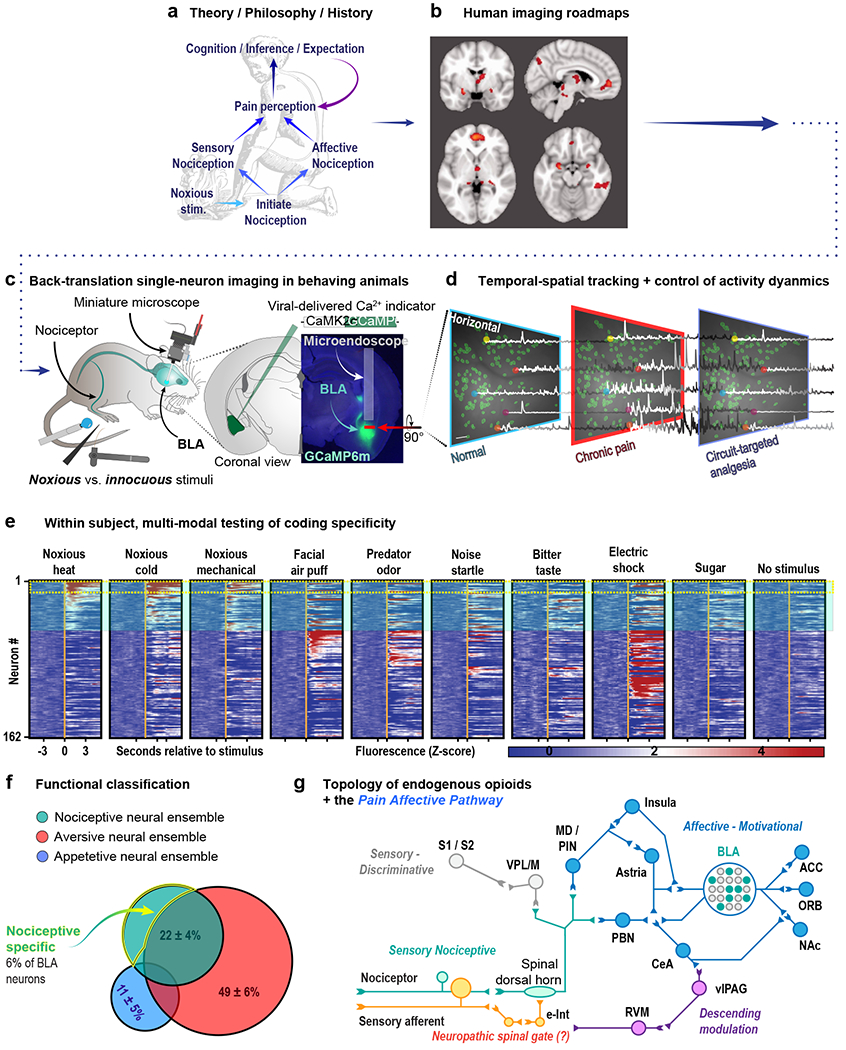
(a) The fundamental groundwork laid forth by historical philosophical and clinical work inform modern investigation of the neural control of pain affect. (b) Human imaging and lesion studies qualitatively and broadly address questions of neuroanatomical function in pain affect. Evidence from historical and modern clinical pain research can provide a starting point from which to identify and interrogate specific neural circuitry at a resolution unachievable with conventional clinical methods. (c) Modern calcium imaging techniques allow for tracking of thousands of individual neurons in awake, behaving rodents at single-cell resolution. This approach has led to, for example, the identification of basolateral amygdala neurons that encode the aversiveness of noxious stimuli (Corder et al., 2019). (d) Endoscopy and two-photon microscopy allow for the simultaneous investigation of genetically defined populations of neurons at high spatial and temporal resolution time-locked to behaviorally significant events (e.g. the application of a noxious stimulus). (e) These techniques reveal the signaling dynamics of individual neurons embedded within neural microcircuitry to be tracked over multiple days in response to numerous stimuli. (f) The resulting information creates a picture of both the role of the circuit as a whole as well as the identification of functional subpopulations within the circuit (g) Ultimately, combining behavioral studies of pain affect informed by clinical findings with circuit-based, single-neuron imaging, and opioidergic cell-type-specific genetic approaches will allow for the most efficient mapping of pain affective pathways embedded within the endogenous opioid system.
