Figure 4. Conserved brain structures involved in pain and analgesia expectation.
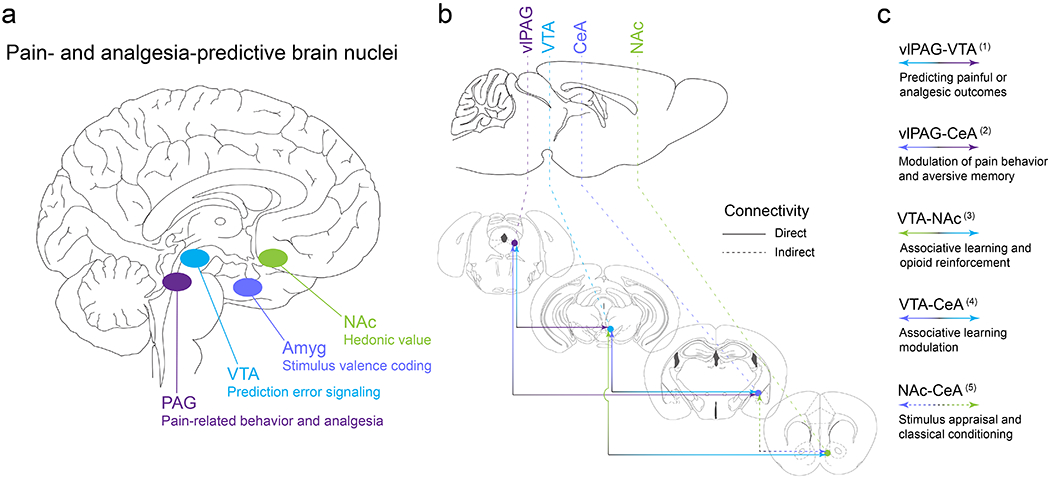
(a) Deep brain stimulation and imaging modalities have revealed nuclei in the human brain that underlie endogenous opioid-mediated analgesia as well as expectancy signals that contribute to pain and the relief of pain. This work provides an excellent foundation for back-translational approaches to identify, target, and manipulate the endogenous opioid circuits that mediate pain relief directly or through learning mechanisms (i.e. placebo analgesia). Here we highlight several critical brain areas, all of which are modulated by endogenous opioid peptides: periaqueductal gray (PAG), ventral tegmental area (VTA), amygdala (Amyg), and nucleus accumbens (NAc). (b-c) The rodent brain shares the same pain-responsive structures. Preclinical models of pain and analgesia have further revealed functional connectivity between nodes and their subnuclei in the distributed pain- and analgesia-predictive neural circuitry. Reciprocal, direct connectivity has been documented between the ventrolateral PAG (vlPAG) and the VTA, the vlPAG and the central nucleus of the amygdala (CeA), the VTA and CeA, as well as the VTA and NAc. Indirect connectivity has been noted for the CeA and NAc. Here we provide broad functional definitions for these neural connections based on ample preclinical studies in animals. (1) vlPAG-VTA (Geisler et al., 2007; Ntamati et al., 2018; Omelchenko & Sesack, 2010; St Laurent et al., 2020; Suckow et al., 2013; Waung et al., 2019); (2) vlPAG-CeA (C. Li et al., 2016; Ozawa et al., 2017; Tovote et al., 2016; Wright & McDannald, 2019); (3) VTA-NAc (Beier et al., 2015; Corre et al., 2018; Klawonn & Malenka, 2018; S. Peciña et al., 2006; Pignatelli & Bonci, 2018; Xia et al., 2011); (4) VTA-CeA (Dedic et al., 2018; Fudge et al., 2017; H. J. Lee et al., 2011; Zhou et al., 2019); (5) NAc-CeA (Ahn & Phillips, 2002; Cardinal et al., 2002; Hall et al., 2001; Zahm et al., 1999).
