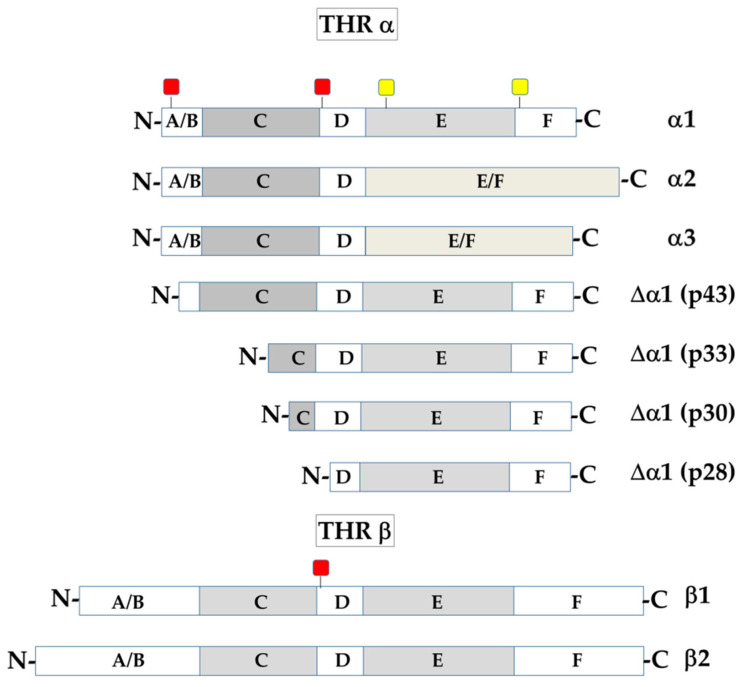
Figure 1.
Schematic representation of the domain structure of THRα and THRβ. The full-length TH receptors contain: (i) an N-terminal A/B domain, involved in transcription transactivation; (ii) a DNA-binding domain (C), which recognizes and binds the TH-response elements (TREs), and is also involved in receptor dimerization; (iii) a hinge domain (D) and (iv) an E/F domain that can be involved in binding the hormone, as well as in protein–protein interactions. The full-length THRα1 contains two nuclear localization signals (NLS: red squares) and two atypical mitochondrial import sequences (MIS: yellow squares). The THRα1 shorter forms, which derive from internal AUG translational usage, lack one or both NLS; in these proteins, the localizing effect of the mitochondrial signals is prevalent. THRβ contains only one NLS (red square) in the D domain [66].