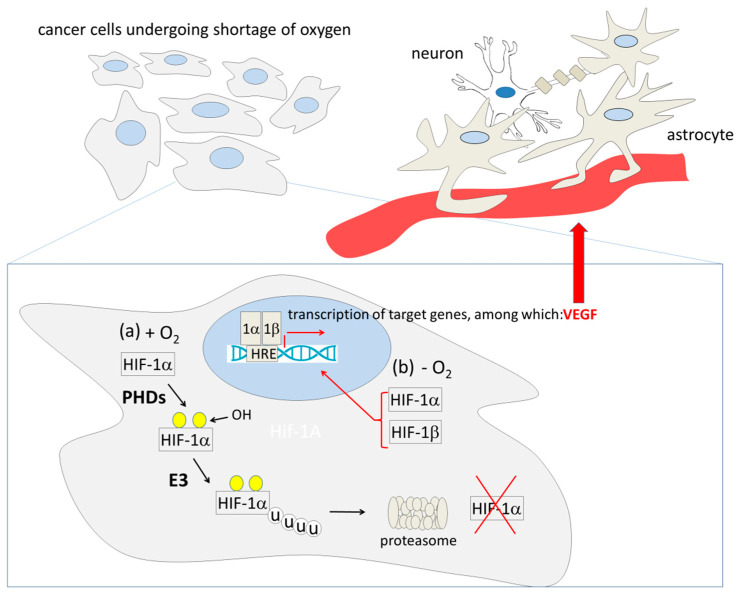
Figure 3.
Dependence of angiogenic processes on oxygen shortage and activation of the hypoxia-induced factor 1 (HIF-1). HIF-1 factor is composed by two subunits, HIF-1α and HIF-1β. In normoxic conditions (a, +O2), HIF-1α is hydroxylated by prolyl hydroxylase domain proteins (PHDs), poly-ubiquitinated (u) by an E3 ligase, and degraded by the proteasome. When cells (including cancer cells) undergo oxygen shortage (b, -O2), PHDs, which use molecular oxygen as a substrate in the hydroxylation reaction, cannot modify any more HIF-1α; as a consequence, HIF-1α is no longer degraded, and combines with HIF-1β; the HIF-1α/HIF-1β dimer enters the nucleus, where it binds to the HIF-1-response elements (HREs) and activates its target genes, including the one encoding vascular endothelial growth factor (VEGF), which in turn stimulates the endothelial cells of the vessels to migrate, proliferate, and differentiate, forming new vessels [273].