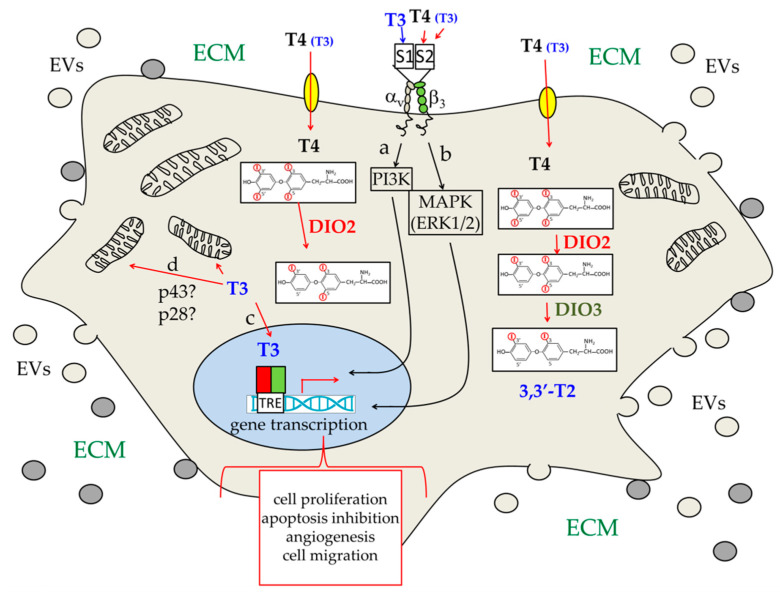
Figure 5.
Schematic view of the different hypothetical pathways triggered by THs in a generic brain cancer cell. THs can enter the cells through membrane transporters (yellow ovals), such as MCT8 and OATP1C1. They can also bind to plasma membrane receptors, the best known of which is αvβ3 integrin. T4 is the main form of circulating THs, and thus the main form present in the extracellular matrix (ECM). As a consequence, T4 is also the most abundant TH that enters the cell, and that is able to bind αvβ3 integrin, at the level of the S2 site (red arrow). T3 is much less concentrated outside the cell but can specifically bind to αvβ3 integrin at the level of the S1 site (blue arrow); it can also bind to the S2 site (red arrow), even if it is with much lower affinity than T4. Binding of T3 to the S1 site elicits a signal transduction pathway (a), which involves the phosphoinositide-3-kinas3 (PI3K). On the other hand, binding of T4 (T3) to the S2 site elicits activation of the MAPK/ERK1/2 pathway (b) [99,100,101,102,103,104,105,106]. T4 that enters the cell is converted to T3 by deiodinase 2 (DIO2). T3 can then enter the nucleus (c) and regulate transcription of target genes both directly, by binding to THRs, and indirectly, by interacting with other regulatory proteins [37,67,89]. The a-c regulatory pathways can all influence, directly or indirectly, the transcription of genes involved in different hallmarks of cancer. In addition, T3 has been suggested to be able to directly regulate mitochondrial activities (d), probably by interacting with ∆α1 proteins, such as p43 and p28 [37,65,66,107,108,109]. Finally, as schematically described in Figure 4, brain cancer cells also release extracellular vesicles (EVs; small light brown circles), which can influence other brain cells in the environment, and also receive EVs from the surrounding cells (grey small circles).