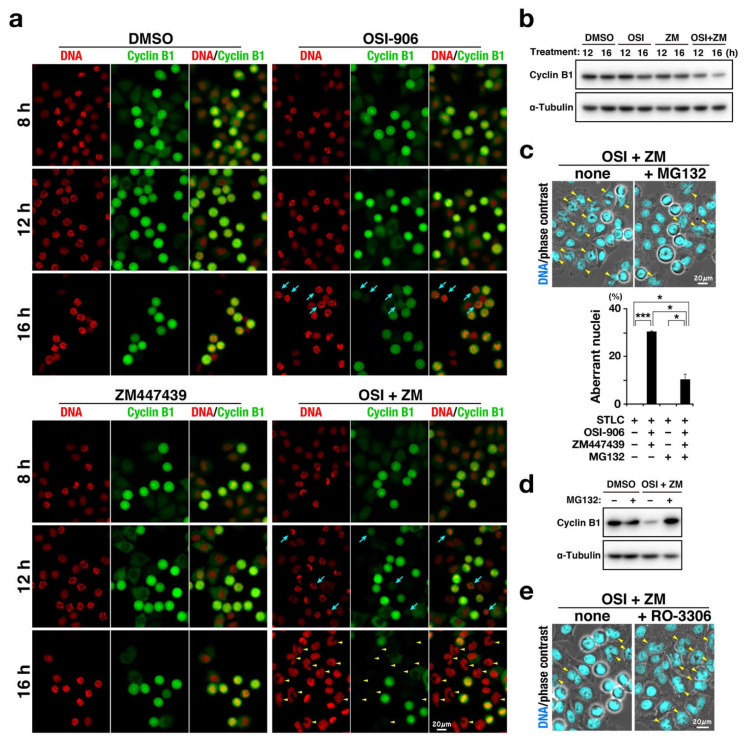
Figure 5.
Combination of OSI-906 and ZM447439 causes mitotic slippage via precocious degradation of cyclin B1. HeLa S3 cells were treated with 7.5 µM S-trityl-L-cysteine in the presence of OSI-906, ZM447439, or their combination (OSI + ZM). Dimethyl sulfoxide was used as solvent control (DMSO). (a) At 8, 12, and 16 h after the start of treatment, cells were fixed and stained for cyclin B1 (green) and DNA (red). Representative images are shown. Blue arrows indicate M phase cells with lower expression levels of cyclin B1. Yellow arrowheads indicate cells with aberrant nuclear morphologies. Scale bar, 20 µm. (b) At 10 h after the treatment, M phase cells were collected by mitotic shake-off and further incubated in microtubes at 37 °C for 2 h and 6 h. Whole cell lysates were analyzed using Western blot with the indicated antibodies. The full blots are shown in Figure S1. (c) At 12 h after the treatment, 40 µM MG-132 was added to the culture. The cells were further incubated for 4 h and stained for DNA (cyan). Yellow arrowheads indicate cells with aberrant nuclear morphologies. Scale bar, 20 µm. The number of cells with aberrant-shaped nuclei was counted and plotted as the mean ± SD calculated from three independent experiments (n > 112). p-values were calculated using the Games–Howell multiple comparison test. * p < 0.05; *** p < 0.001. (d) At 10 h after the treatment, M phase cells were collected by mitotic shake-off and further incubated in microtubes at 37 °C for 6 h. During the last 4 h of incubation in microtubes, the cells were treated with 40 µM MG-132. Whole cell lysates were analyzed using Western blot with the indicated antibodies. The full blots are shown in Figure S1. (e) At 10 h after the treatment, 6 µM RO-3306 was added to the culture. The cells were further incubated for 2 h and stained for DNA (cyan). Yellow arrowheads indicate cells with aberrant nuclear morphologies. Scale bar, 20 µm.