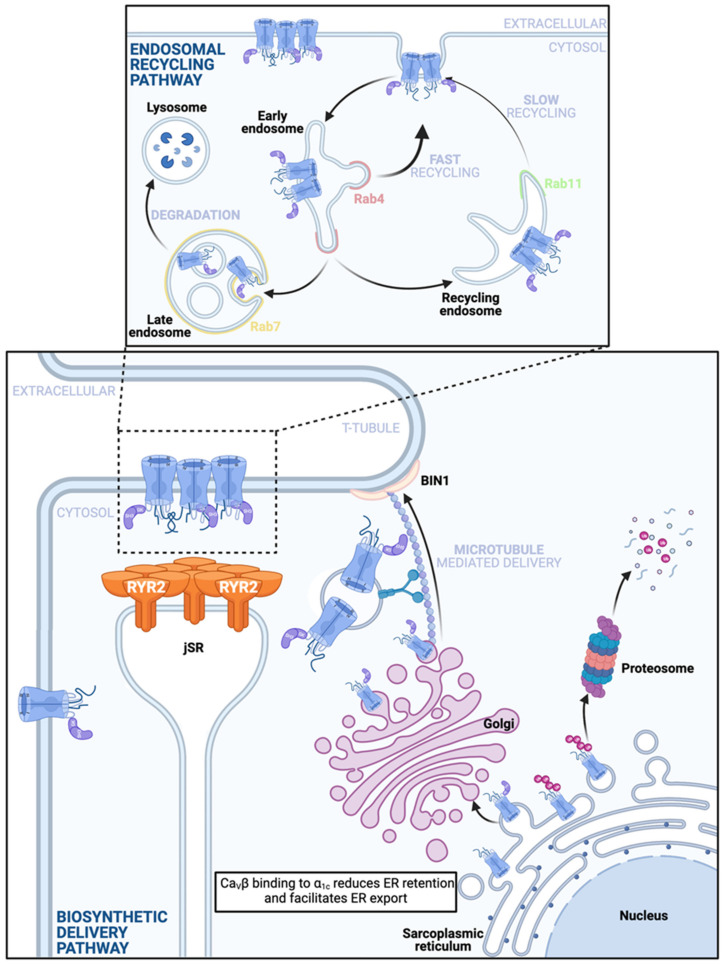
Figure 2.
Anterograde CaV1.2 channel delivery pathways. Bottom: A depiction of the ‘Biosynthetic Delivery Pathway’ that commences when CaVα1c are translated directly into the rER membrane. These pore-forming subunits have several ER retention motifs and just one identified ER export motif. Binding of the CaVβ subunit to the CaVα1c while in the ER membrane is thought to lessen the influence of the retention signals and favor channel export, whereby the channels subsequently exit the ER in vesicles to the Golgi complex. Absence of the CaVβ subunit may render the channels more vulnerable to ubiquitination on the ER membrane, precipitating proteosomal degradation. Note this vulnerability has been reported in neuronal CaV1.2 and has not yet been confirmed in the cardiac channels. Single or clustered channels exit the trans-Golgi network (TGN) and are carried along microtubules by motor proteins to BIN1-anchored delivery hubs on the t-tubule sarcolemma to assume positions in dyadic and caveolar regions. It is unclear exactly when and where in the anterograde trafficking pathway CaVα2δ joins the complex. Top: Stable sarcolemmal CaV1.2 channel expression is maintained in the face of ongoing channel internalization by the endosomal recycling pathway. Endocytosed channels enter the early endosome and are sorted, a subpopulation is sent to Rab7 positive late endosomes and may subsequently undergo lysosomal degradation. The remainder of the channels are recycled back to the membrane either through a fast, direct pathway from the early endosome along a Rab4-dependent fast recycling pathway, or through a slower Rab11-dependent recycling pathway from the recycling endosome. If some channels are degraded, it is possible that endosomal reservoirs of channels take their place and supplement this pathway to maintain stable membrane expression.