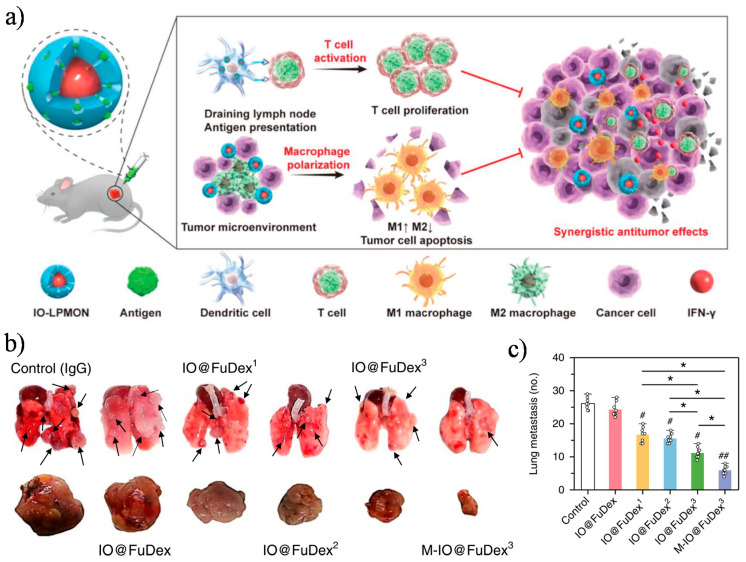
Figure 7.
(a) Schematic representation of iron oxide-embedded, large-pore mesoporous organosilica nanospheres (IO-LPMONs) loaded with OVA protein. OVA-loaded IO-LPMONs are taken up by dendritic cells, which upon activation migrate to the draining lymph nodes and trigger the initiation of specific immune responses. Simultaneously, the nanovector acts not only as a carrier, but also as an immunomodulator, promoting the repolarization of TAMs from an M2 to M1 profile. (b) The photographs of dissected breast primary tumours and metastatic lungs from mice treated with the various IO@FuDex nanoformulations (IO©FuDex, no antidobies; IO@FuDex1, only anti-CD3/CD28 antibodies; IO©FuDex2, only anti-PD-L1 antibody; IO@FuDex3, anti-CD3/CD28 and anti-PD-L1 antibodies; and M-IO@FuDex3, anti-CD3/CD28, and anti-PD-L1 antibodies + magnetic navigation). The arrows point the metastatic nodules in the lungs. (c) Quantification of the metastatic nodules confirm that MNPs can support the synergistic anti-tumour effect of checkpoint inhibitors and T cell activators, and that of the therapeutic efficacy of IO@FuDex3 by promoting specific localization of the therapeutics with magnetic navigation (b). Reproduced with permission from References [116,138] (Copyright 2019 Wiley-VCH and Copyright 2018 Nature Publishing Group).