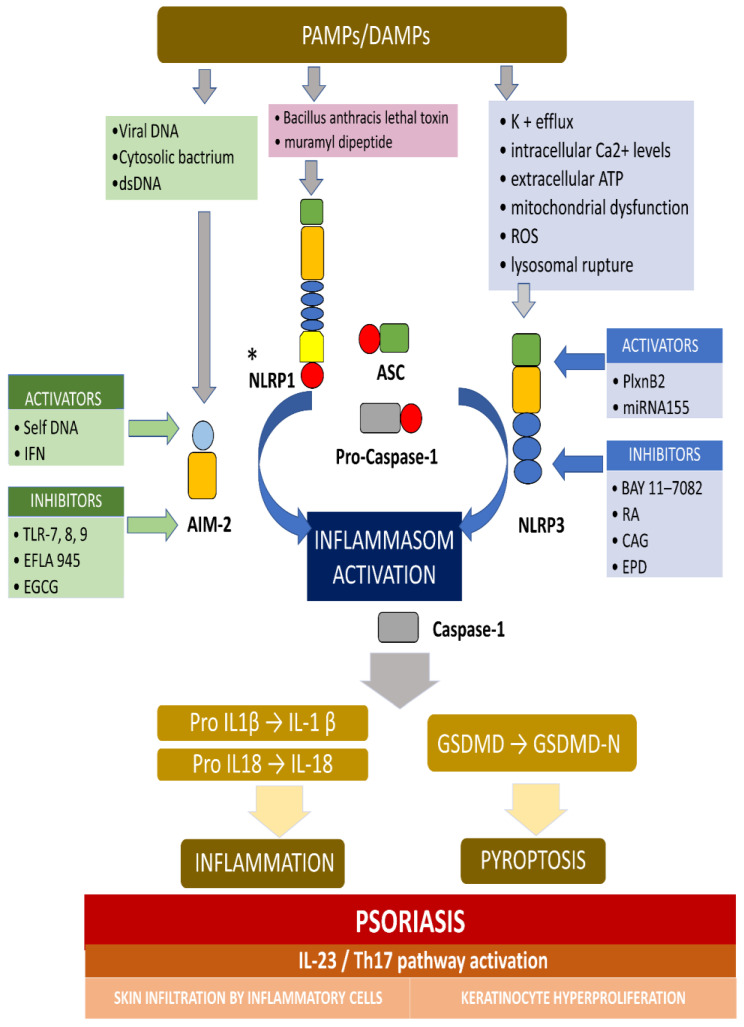
Figure 2.
The activation and inhibition of the AIM2 and NLRP3 inflammasome in psoriasis. * Activators and inhibitors are not reported for NLRP1. The AIM2 inflammasome detects cytosolic dsDNA, including DNA viruses and cytosolic bacterium, which causes inflammasome activation. The NLRP3 inflammasome can be activated by a variety of PAMPs and DAMPs, which cause, for example, potassium efflux, extracellular ATP, lysosomal destabilization, intracellular calcium levels, and ROS (reactive oxygen species). When the main components of the inflammasome is connecting and the active inflammasome is formed, it directly recruits and cleaves pro-caspase1 into active caspase-1, which proteolytically activates the pro-inflammatory cytokines IL-1b and IL-18. In addition, the activated inflammasome cleaves gasdermin D (GSDMD) into active N-terminal fragment (GSDMD-N), which drives a lytic type of cell death pyroptosis. In psoriasis, the self-DNA in patients, and IFN in keratinocyte in mouse activate AIM-2 inflammasome, while TLR-7/8/9 (toll-like receptor), EFLA 945 (red vine leaf extract), and EGCG (epigallocatechin-3-gallate) can inhibit the AIM2 inflammasome. In turn, PlxnB2/ligand and miRNA155 activate the NLRP3 inflammasome, while BAY 11-7082, rosmarinic acid (RA), cycloastragenol (CAG) and EPD (the effective part of Datura metel L.) inhibit this inflammasome. The active IL-1b and IL-18 activates IL23/Th17 pathway, inducing a numerous of inflammatory cytokines and chemokines. Different kinds of immune cells infiltrating into the skin and, finally, causing hyperproliferation in the epidermis, was observed.