Table 2.
Preliminary results in the assessment of the therapeutic role of exosomes in patients with COVID-19. EVs: extracellular vesicles; MVs: microvesicles; PMVs: platelet-derived MVs; PaO2/FiO2 ratio: arterial pressure of oxygen/inspired fraction of oxygen ratio;  increase;
increase; 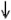 decrease.
decrease.
| Employing Extracellular Vesicles (EVs) as Therapeutic Agents in COVID-19 | |||
|---|---|---|---|
| Administration of MSC-Derived EVs to Patients | Improved Effects | EV Effects in COVID-19 Patients | References |
| MSC-derived exosomes administered i.v. to 24 COVID-19 patients with moderate to severe pathology |
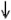 CRP/ferritin/D-dimer levels CRP/ferritin/D-dimer levels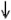 neutrophil number neutrophil number CD3+/CD4+/CD8+ lymphocyte number CD3+/CD4+/CD8+ lymphocyte number PaO2/FiO2 ratio PaO2/FiO2 ratio |
|
[164] |
| MSC-derived exosomes administered by aerosol inhalation to COVID-19 patients: 5 days to 24 patients; twice a day, 10 days to 30 patients | No official results yet | ClinicalTrials.gov: NCT04276987/ NCT04491240/ NCT04602442 |
|
| Engineered exosomes overexpressing CD24, aerosolized by inhalation, once a day, 5 days to 35 COVID-19 patients | suppress cytokine storm |
|
ClinicalTrials.gov: NCT04747574 |
