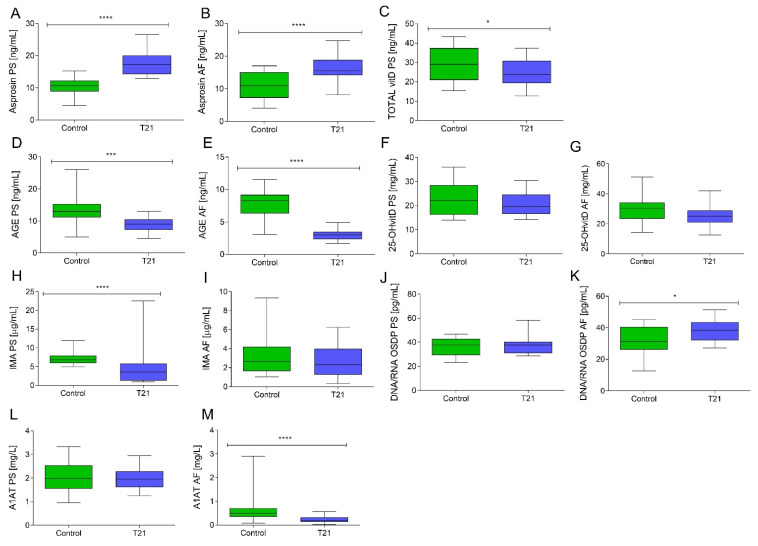
Figure 1.
The studied protein concentrations measured in the plasma and amniotic fluid samples. Different asterisks above the bars indicate significant differences compared to the control (* p ≤ 0.05; *** p ≤ 0.001; **** p ≤ 0.0001). (A) Plasma asprosin; (B) amniotic fluid asprosin; (C) plasma total vitamin D; (D) plasma advanced glycation end products; (E) amniotic fluid advanced glycation end products; (F) plasma 25-OH vitamin D; (G) amniotic fluid 25-OH vitamin D; (H) plasma ischemia-modified albumin; (I) amniotic fluid ischemia-modified albumin; (J) plasma DNA/RNA oxidative stress damage product; (K) amniotic fluid DNA/RNA oxidative stress damage products; (L) plasma alfa-1-antitrypsin; (M) amniotic fluid alfa-1-antitrypsin. A1AT, alfa-1-antitrypsin; AF, amniotic fluid; AGE, advanced glycation end products; Control, control group; IMA, ischemia-modified albumin; OSDP, oxidative stress damage product; PS, plasma; T21, trisomy 21.