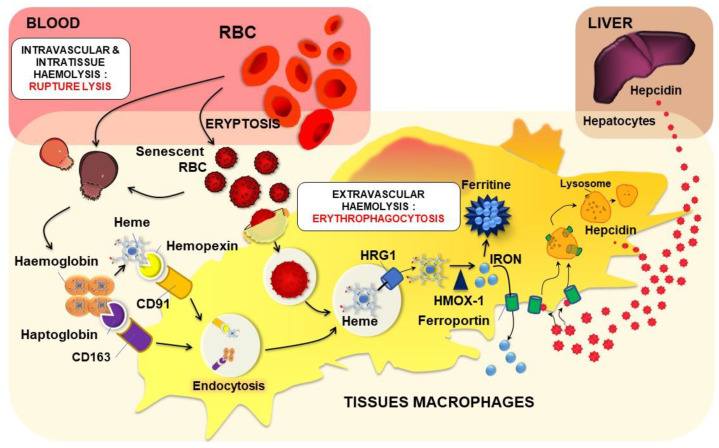
Figure 5.
Erythrocyte haemolysis and heme iron recycling. Modifications (oxidative stress, glycation; see chapter IV) and/or eryptosis can trigger the lysis of circulating or extravasated erythrocytes in some tissues. After such intravascular or intratissue haemolysis, haptoglobin and hemopexin bind to haemoglobin and heme, respectively. Complexes are recognised by macrophages and endocytosed via their specific receptors CD163 for haemoglobin/haptoglobin complexes and CD91 for heme/hemopexin complexes. In the case of EP of senescent or eryptotic erythrocytes (intracellular haemolysis), specific recognition of old erythrocytes is performed by tissue macrophages, and a phagolysosome containing the old erythrocyte is formed. Both CD163- and CD91-mediated endocytosis and EP lead to the formation of a vacuole containing heme. Heme is then transported to the cytosol via HRG1 and degraded by HMOX1 (Heme oxygenase 1) to produce iron. According to the body needs, such iron is either stored in ferritin or transported outside the cells via the transporter ferroportin. Hepcidin, a small but powerful regulatory peptide of iron homeostasis, is mainly produced by hepatocytes and to a lesser extent by immune cells such as macrophages. Hepcidin binds to ferroportin inducing its internalization and degradation. Such interaction is primordial for regulating macrophage iron levels as well as systemic iron homeostasis.