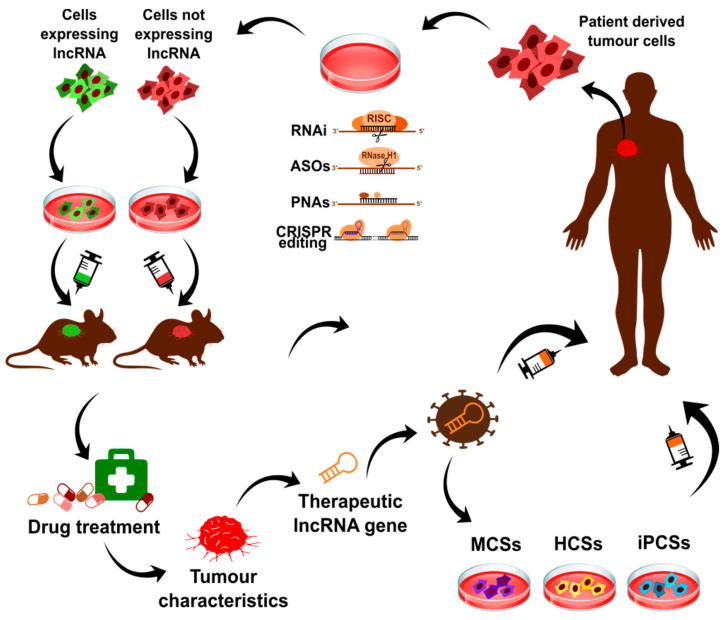
Figure 6.
Schematic of anti-tumour gene therapy approaches involving lncRNA. The altered expression of lncRNA is widely observed in tumour tissues. The initial step for gene therapy includes collecting and growing tumour cells that are obtained from the patient (or from commercial cell line collections). The next step is testing the function of lncRNA of interest in tumour, which might be done with cell-editing assays using targeting lncRNA by RNAi, ASOs, PNAs or CRISPR/Cas9. Resulted cell lines with altered expression of lncRNA are characterized in line with unmodified tumour cells. The cell-based experiments include analysis of cell viability, proliferation, migratory potential, response to therapeutic compounds. The obtained results need to be confirmed in vivo for example after injection of lncRNA expressing cells/lncRNA-silenced cells to immunodeficient mouse (xenograft models). The tumour growing in xenografts mimics the patient’s tumour. The characteristics of tumour mass, growth rate and specific aspects of its behavior, such as assessing metastatic growth, is needed to validate the therapeutic potential of lncRNA. Additionally, xenografts might be subjected to anti-tumour therapy to test response to drugs. The verified therapeutic lncRNA gene might be then encapsuled with the non-immunogenic vectors like viruses and injected to the patient. Ultimately, stem cell lines such as MCSs (mesenchymal stem cells), HCSs (haematopoietic stem cells) or iPCSs (induced pluripotent stem cells) might be transfected with the lncRNA to obtain cells expressing lncRNA. The injection of these modified cells to the patient increases the ability to generate healthy cells. Several lncRNAs tested for gene therapy are described within the text. This is an original figure.