Table 4.
Selected autophagy activators under clinical investigation.
| Autophagy Activator | Chemical Structure | Study Type | References |
|---|---|---|---|
| Rapamycin |
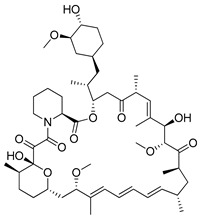
|
Clinical trials: therapy of kaposiform hemangioendothelioma in children, bladder cancer, HER2+ metastatic breast cancer, refractory solid tumors, NSCLC and pediatric relapsed or refractory tumors |
NCT04077515, NCT02753309, NCT04375813, NCT04736589, NCT02688881, NCT04348292, NCT02574728 |
| Temsirolimus |
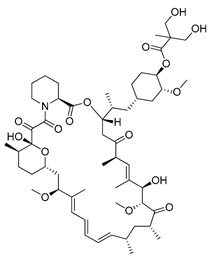
|
Preclinical studies: colorectal cancer, prostate cancer, human papillomavirus-related oropharyngeal squamous cell carcinoma or advanced solid tumors treatment Clinical trials: advanced/metastatic malignancies, gynecological malignancies, rare tumors, diffuse intrinsic pontine glioma or solid tumors therapy |
[194,195,196,197] NCT01552434, NCT01065662, NCT01396408, NCT02420613, NCT01375829 |
| Everolimus |
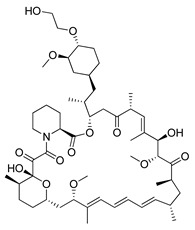
|
Preclinical studies: treatment of advanced papillary variant renal cell carcinoma, triple-negative breast cancer or advanced solid tumors Clinical trials: recurrent or progressive ependymoma in children, Hodgkin lymphoma, metastatic transitional cell carcinoma of the urothelium, advanced gynecologic malignancies and breast cancers or recurrent low grade gliomas in young adults and pediatric patients |
[199,200,201] NCT02155920, NCT03697408, NCT00805129, NCT03154281, NCT04485559 |
| Metformin |
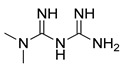
|
Clinical trials: breast cancer, colon cancer, thoracic neoplasm or prostate cancer therapy |
NCT04559308, NCT04387630, NCT01980823, NCT04741204, NCT03359681, NCT03477162, NCT02176161, NCT02339168 |
