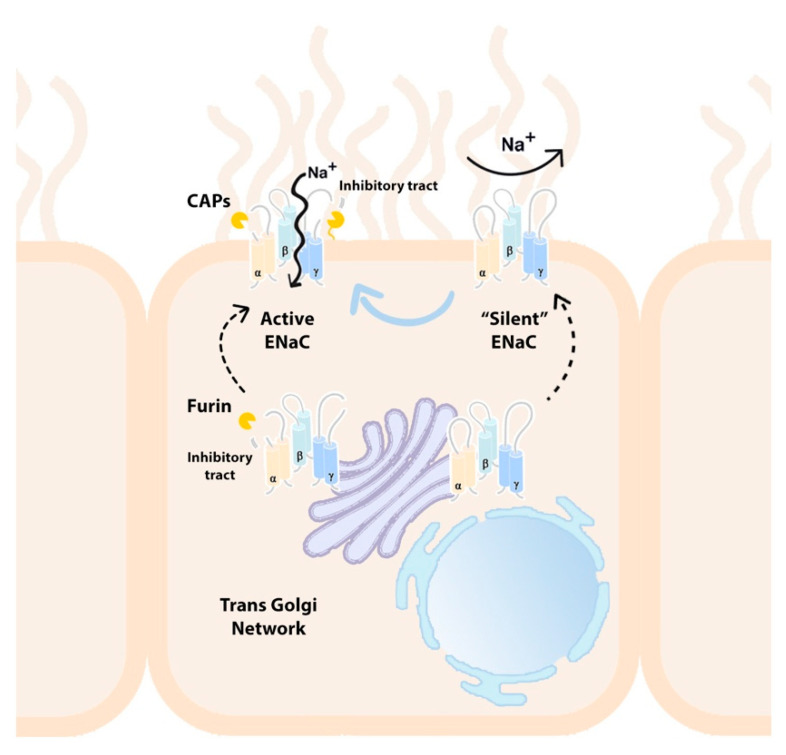
Figure 2.
ENaC is a heterotrimeric structure composed of α, β and γ subunits at the apical surface of the airway epithelium. Newly synthesised ENaC is trafficked intracellularly through the trans-Golgi network (TGN) where it is subject to cleavage by furin at two specific sites in the α subunit, which results in the release of a peptide inhibitory tract. Partial cleavage of one site in the γ subunit by furin also takes place. The γ subunit requires further processing at the cell surface by channel activating proteases (CAPs), some of which are TLPs, at a site distal to the furin cleavage site in order to fully activate ENaC through the release of a second inhibitory fragment. Alternatively, a subpopulation of channels known as “silent” ENaC are able to bypass furin processing in the TGN and move directly to the cell membrane where they show minimal activity until they are cleaved by soluble or membrane-bound CAPs.