Abstract
The unique electron deficiency and coordination property of boron led to a wide range of applications in chemistry, energy research, materials science and the life sciences. The use of boron-containing compounds as pharmaceutical agents has a long history, and recent developments have produced encouraging strides. Boron agents have been used for both radiotherapy and chemotherapy. In radiotherapy, boron neutron capture therapy (BNCT) has been investigated to treat various types of tumors, such as glioblastoma multiforme (GBM) of brain, head and neck tumors, etc. Boron agents playing essential roles in such treatments and other well-established areas have been discussed elsewhere. Organoboron compounds used to treat various diseases besides tumor treatments through BNCT technology have also marked an important milestone. Following the clinical introduction of bortezomib as an anti-cancer agent, benzoxaborole drugs, tavaborole and crisaborole, have been approved for clinical use in the treatments of onychomycosis and atopic dermatitis. Some heterocyclic organoboron compounds represent potentially promising candidates for anti-infective drugs. This review highlights the clinical applications and perspectives of organoboron compounds with the natural boron atoms in disease treatments without neutron irradiation. The main topic focuses on the therapeutic applications of organoboron compounds in the diseases of tuberculosis and antifungal activity, malaria, neglected tropical diseases and cryptosporidiosis and toxoplasmosis.
Keywords: organoboron compound, anti-cancer drug, anti-tuberculosis, anti-malaria drug, neglected tropical disease, crypto and toxoplasmosis treatment
1. Introduction
Many infectious diseases are caused by microorganisms, such as tuberculosis and malaria, and the current treatments for them are unsatisfactory as there are a few or no suitable drugs. Four types of such frequently occurring diseases, in which organoboron compounds have already shown high potential as acceptable drug agents, have been selected to survey in this review. The four common diseases are tuberculosis, malaria, neglected tropical diseases, and the parasitic diseases of cryptosporidiosis and toxoplasmosis, and they are briefly introduced as follows. (1) Tuberculosis is an infectious disease caused by Mycobacterium tuberculosis, which has a high level of mortality worldwide and has already gained resistance to first- and second-line therapy [1]. (2) Malaria is a disease caused by the Plasmodium parasite and accounts for one of the leading causes of death worldwide despite decades of strategic interventions aimed at reducing incidence and mortality [2]. (3) Neglected tropical diseases (NTDs) are a group of twenty highly parasitic (fungi, protozoa, helminths or metazoan worms), viral and bacterial diseases as classified by the World Health Organization (WHO) [2,3,4]. NTDs affect more than one billion people, especially children, and prevail in poor populations living in tropical and subtropical climates, causing a huge toll in terms of morbidity and mortality, as well as public economies [2,3,4], and (4) cryptosporidiosis and toxoplasmosis are other dangerous diseases caused by important protozoan pathogens of humans, while Cryptosporidium is a common cause of moderate-to-severe diarrhea in children under five years of age [5].
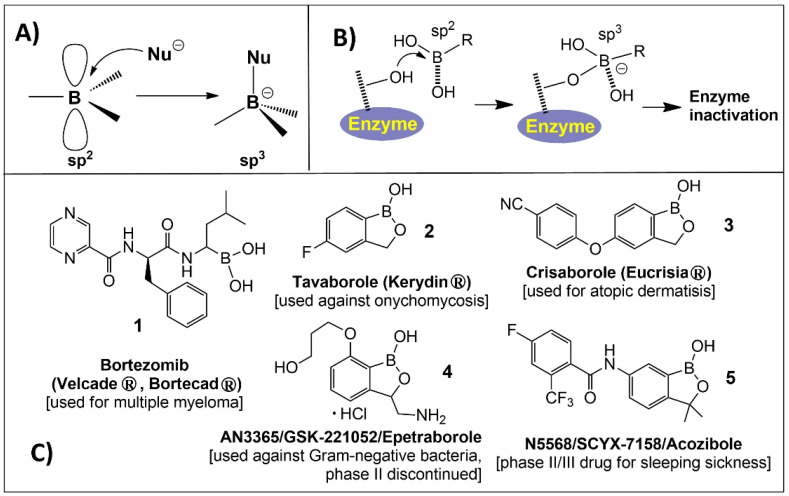
Boron has a wide range of applications in chemistry, energy research, materials science and the life sciences [6,7,8,9,10,11]. The use of organoboron compounds as medication agents has a long history. For example, boron compounds, 4-borono-L-phenylalanine (BPA) and sodium borocaptate (BSH), have been used as boron carriers in boron neutron capture therapy (BNCT) for decades to treat various tumors, such as malignant brain tumor and melanoma [8]. Nevertheless, the updating of BNCT is beyond the scope of this review. In the beginning of the 20th century, many scientists concentrated their attention on the development of boron-based organic chemistry [9]. Cluster-based boron compounds are in the latest class that takes advantage of the properties of many boron atoms in the cage [10,11], including their unique electronic properties and ability to form covalent bonds in organoboron compounds, which make them a suitable agent for drug discovery. Boron compounds are electrophiles (strong Lewis acids) due to their empty p-orbital. When accepting a pair of electrons from a nucleophile (Lewis base), they easily inter-convert from the trigonal sp2 to the tetrahedral sp3 hybridization states, as shown in Figure 1A. The use of organoboron compounds as enzyme inhibitors is mostly based on this easy conversion (Figure 1B) [12]. After decades of studies, numerous bioactive molecules and molecular tools containing single boron atoms were developed [13]. Bortezomib, 1 (PS-341), (Figure 1C) trade name Velcade, from Takeda Pharmaceutical, is a dipeptide boronic acid (peptidomimetic), and it was approved by the FDA in 2003 for the clinical treatment of multiple myeloma [14]. Benzoxaboroles acquired reputation in medicinal chemistry only as of 2006, when 5-fluorobenzoxaborole (Tavaborole, AN2690, 2 in Figure 1C,) showed antifungal action [15], and its topical solution (Kerydin®) was approved by the FDA in 2014 for the treatment of onychomycosis [16]. Another benzoxaborole approved by the FDA in December 2016 was Crisaborole 3 (Figure 1C), which is traded in the USA under the name of EUCRISA® for clinical use in the treatment of mild-to-moderate atopic dermatitis [17]. Currently, it is the first and only non-steroidal in anti-inflammatory monotherapy as the phosphodiesterase type 4 inhibitor, commonly referred to as a PDE4 inhibitor for the skin. Benzoxaboroles possess unique chemical properties, such as remarkable chemical stability, low toxicity, ease in synthesis, and high targeting specificity. These attributes make them very attractive therapeutic agents [18]. In addition, several organoboron compounds also demonstrate strong antibacterial activity, specifically against the enteric group of Gram-negative bacteria. In this context, a promising example of an antibacterial oxaborole-based species is the chiral benzoxaborole 4 (AN3365/GSK2251052) (Figure 1C) [19]. Compound SCYX-7158/AN5568 (5, in Figure 1C) is identified as a promising agent for Human African trypanosomiasis (HAT) and has entered clinical phase II/III evaluation. Earlier observations of anti-fungal, anti-bacterial, and anti-inflammatory activities of benzoxaboroles and other organoboron compounds represented the key result that led to the discovery of their potential for the treatment of various infectious diseases [20]. This review will focus on the particular type of bioactivity of organoboron compounds covering the medicinal applications in infectious disease caused by protozoa, fungi and helminths, describing progress in drug development, cytotoxicity and the proposed mechanisms of action. Other organoboron compound-based antibacterial or antiviral drugs have been reviewed elsewhere [21,22]. Thus, the review covers four areas of therapeutic applications of organoboron compounds: tuberculosis and antifungal activity, malaria, neglected tropical diseases and cryptosporidiosis and toxoplasmosis.
Figure 1.
(A) Boron electronic features and configurational modification of boron; (B) Mechanism of action of boron-based compounds for enzyme inhibition; (C) Examples of reported boron compounds and marketed benzoxazole drugs.
2. Tuberculosis and Antifungal Activity
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), is a highly contagious chronic bacterial infection and is one of the top 10 causes of death worldwide [23]. In 2019, more than 10 million people fell ill with TB, and around 1.4 million died from the disease [23]. The Mtb is transmitted by aerosol and infection occurs when a person inhales droplet nuclei containing tubercle bacilli that reach the alveoli of the lungs. These tubercle bacilli are ingested by alveolar macrophages and destroyed or inhibited. If the bacilli remain alive, they may spread by way of lymphatic channels or the bloodstream to other tissues and organs (brain, larynx, lymph node, lung, spine, bone, or kidney). Within 2 to 8 weeks, special immune cells called macrophages ingest and surround the tubercle bacilli. The cells form a barrier shell (granuloma) that keeps the bacilli contained and under control. If the immune system cannot keep the tubercle bacilli under control, the bacilli begin to multiply rapidly (TB disease) [24]. Worldwide, in 2019, close to half a million people developed rifampicin-resistant TB (RR-TB), of which 78% had multidrug-resistant TB (MDR-TB) [23]. MDR-TB is treatable and curable by using second-line drugs. However, second-line treatment (kanamycin, amikacyn) options are limited, and they require extensive chemotherapy (up to 2 years of treatment) with medicines that are expensive and toxic [25]. In this regard, many efforts have been dedicated to the discovery and development of new anti-TB agents with new mechanisms of action to control drug-resistant disease [26]. The most active frontiers are surviving as follows.
2.1. Benzoxaboroles
1,3-Dihydro-1-hydroxyl-2,1-benzoxaboroles (or dihydrobenzoxaborole or benzoboroxoles) were first synthesized and characterized in 1957 by Torssell [27]. After the discovery that ortho-hydroxyalkyl arylboronic acids can form a complex with glycosides under physiologically relevant conditions, they have been investigated as molecular receptors for sugars and glycoconjugates, in supramolecular chemistry and as building blocks and protecting groups in organic synthesis [28]. Reviews describing these applications of benzoxaboroles were recently published [29,30].
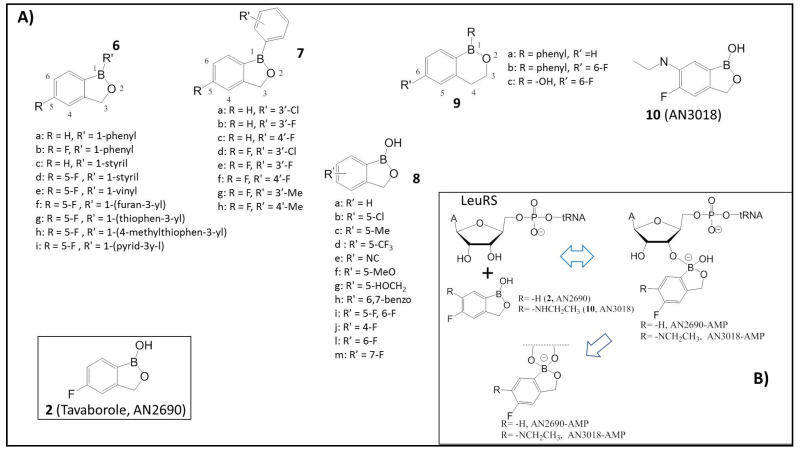
The dihydrobenzoxaboroles bearing aryl, heteroaryl, or vinyl substituents at the 1-position (6a–i), as shown in Figure 2, were reported [29,30,31,32,33]. These substitutions showed equal or decreased activity against fungi. The first lead compound was 1-phenyldihydrobenzoxaborole, 6a, which showed weak activity on a broad spectrum of fungi with minimum inhibitory concentration (MIC) values of 4–8 μg/mL. The following substitution to 5-fluoro-substituted benzoxaborole 6b led to a 2- to 8-fold increase in antifungal activity. Starting from compound 6a to determine the effect of hydrophobicity, many derivatives with various substitutions of R′ in the phenyl ring in position 1 (1-phenyldihydrobenzoxaborole 7a-h) (Figure 2A) were synthesized.
Figure 2.
(A) Schematic representations of benzoxaborole compounds 2 (AN2690) and 6–10; (B) Proposed reaction mechanism of 2 and 10 (AN3018) on leucyl tRNA synthetase (LeuRS) resulting in spiro-product inhibitor: The sp2 hybridized boron atom possesses an empty p-orbital that accepts electrons from the hydroxyl groups of the terminal adenosine and forms an adduct with the tRNA (Adapted from [31,32]).
To enhance hydrophilicity, the 1-phenyl group was replaced with a 1-hydroxy group to prepare 1-hydroxydihydrobenzoxaboroles (8a), as per the published report. Compound 8a showed an 8-fold increase in activity against C. neoformans, and 2 (AN2690) showed an 8-fold increase in activity against A. fumigatus, respectively [29,30,31,32,33]. To determine the structure–activity relationship of this scaffold, the 5-F group was substituted with other groups (8b–m). The results showed that 2 (R′ −F) and 8b (AN2718, R′ −Cl) are the most active derivatives. The 5-chloro-substituted benzoxaborole 8b (AN2718) is being developed now by Anacor pharmaceutical, a company pioneering the field of boron compounds, for the topical treatment of tinea pedis, dermatophyte fungal infection of the soles of the feet and the interdigital spaces [29,30,31,32,33]. The ring size increase from a five-membered oxaborole of 6a, 6b, and 2 to the corresponding six-membered oxaborin 9a, 9b and 9c showed that 1-phenyl substituted oxaborin 9a and the 5-fluoro-1-phenyloxaborin 9b were approximately 2-fold and 4- to 16-fold less active than the oxaborole 6a and 6b, respectively [29,30,31,32,33].
Compound 2 is the most active against dermatophytes T. rubrum and T. mentagrophytes, which are the primary fungal pathogens that cause onychomycosis [31]. The FDA approved the application of 2 (Tavaborole, AN2690) in 2014 as the first oxaborole-based antifungal new drug for the topical treatment of onychomycosis of the toenails [34]. The mechanism involves Tavaborole 2 forming a covalent adduct with the 3′-adenosine of tRNA and inhibiting leucyl-tRNA synthetase (LeuRS) (IC50 2.1 μM) (Figure 2B) [32]. LeuRS belongs to aminoacyl-tRNA synthetases (aaRS), a class of enzymes which are crucial for gene translation. It also plays a critical role in protein synthesis by catalyzing the specific amino acid attachments to their corresponding tRNA during the translation of the genetic code. Many aaRS enzymes possess a proofreading (editing) mechanism that hydrolyzes tRNAs’ aminoacylated functionality with the incorrect amino acid [32]. Thus, LeuRS is a proofreading aaRS, which possesses distinct synthetic aminoacylation and editing active sites separated by more than 30Å. The aminoacylation reaction occurs in two steps: the formation of an enzyme-bound aminoacyl-adenylate (I), followed by the transfer of this activated amino acid to either the 2′- or 3′-hydroxy group on the 3′-terminal adenosine of tRNA (II) [32]. The inhibition of either one of these enzymatic stages (I, II) leads to the accumulation of uncharged tRNA molecules, which bind to ribosomes, causing the interruption of polypeptide chain elongation [32]. These enzymes have been a focus of antimicrobial research as potential targets for more than a decade [35]. Seiradake et al. determined the structure of the C. albicans editing domain complex with compound 10 (AN3018, 6-ethylamino analogue of 2, Figure 2) to provide a structural basis for designing and enhancing the specificity of these benzoxaborole antifungals [36].
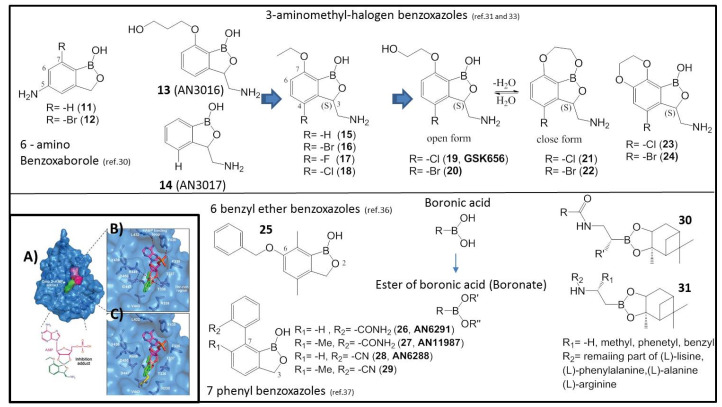
The 6-aminobenzoboroxoles have also been synthesized and found to be non-toxic [37] in general. The derivatives of 11 and 12 with primary amino groups showed good antimycobacterial activity against Mtb H37Rv (11, MIC 1.9 μM, 12 MIC 15.6 μM) [37]. The study identified two lead compounds of 11 and 12, which urges their further development. In the course of an initial drug screening, conducted in Anacor Pharmaceuticals, benzoxaboroles 13 (AN3016) and 14 (AN3017) were found to provide low MIC against Mtb H37Rv (13, 1 μg/mL and 14, 1.8 μg/mL) and inhibitory activity towards Mtb LeuRS (IC50: 13, 3.5 μM; 14, 0.64 μM) [38]. The incorporation of 3-aminomethyl and 7-ethoxy moieties into one molecular structure to form compound 15 showed an increase in activity (15, MIC 0.13 μg/mL, Mtb LeuRS IC50 0.13 μM) [38]. To improve the bio-capacity of 15, structural and biophysical studies were performed through pharmacokinetic investigation to understand its binding mode to Mtb LeuRS. Crystallization with different editing domain constructs of Mtb LeuRS was attempted in the presence of compound 15 with AMP. The boron atom in compound 15 forms a bidentate covalent adduct with AMP (Figure 3A), which mimics the terminal nucleotide Ade76 of the tRNA acceptor. The amino acid residues of T336 to T337 of the threonine-rich region provide multiple H-bonding interactions to the covalent adduct, and L432 and Y435 of the AMP binding loop have extensive H-bonding and hydrophobic contacts with AMP (Figure 3B) [38]. In addition, the amino group of compound 15 builds three key interactions with the carboxylic acid side chains of D447 and D450 and the carbonyl group of M441. The 7-ethoxy substitution not only enables a new interaction with R449 but also packs with the Ade76 ribose, thus further stabilizing the boron-tRNA adduct (Figure 3B) [38]. The superposition of the adduct-bound structure of 15 with that of the E. coli LeuRS editing with the methionine-bound domain shows that the 3-aminomethyl benzoxaborole moiety occupies the same position as the non-cognate amino acid (Figure 3C) [39]. A series of 3-aminomethy benzoxaboroles were evaluated as Mtb LeuRS inhibitors, and most of them were produced and tested as a race mate first, and later separated into the active (S)-isomer. In general, the (S)-enantiomer is more potent compared to the race mate or A (R)-isomer [38]. Thus, the most potent analogs were compounds 16–18 with halogen (Br, F, Cl, (Figure 3)) substitutions at 4-position. These compounds showed an increase in activity against Mtb H37RV (MIC 0.02–0.05 μM), an increase in potency towards Mtb LeuRS (IC50 0.06–0.08 μM) and, therefore, they were selected for in vivo murine pharmacokinetic analysis. All three compounds were very efficacious, in both the acute and chronic mouse Mtb models, with a potency comparable to that of the frontline drug isoniazid [38].
Figure 3.
Structures of benzoxaborole compounds 11–31. (A) X-ray cocrystal structure of LeuRS with compound 15. Crystal structure of the Mtb LeuRS editing domain in complex with compound 15 (carbon atoms are shown in green) and AMP (carbon atoms are shown in magenta); (B) Zoomed view into the editing site of M. tuberculosis LeuRS showing the compound 15-AMP adduct; (C) Overlay of the LeuRS editing domain of Mtb and E. coli in complex with methionine (in yellow). The 3-aminomethyl group of compound 15 mimics the amino group of methionine, including the interaction with the bacterium-specific residue D447. (Adapted from [36,37,38,40,45]).
One of the major drawbacks of the series was its potential toxicity. In order to improve the selectivity of the Mtb LeuRS inhibitors, further studies were performed. First, lipophilicity optimization of the sidechain was investigated by incorporating aromatic moieties to the 7-alkoxyl group, but these derivatives showed a reduction or loss of antitubercular activity and a decrease in Mtb LeuRS potency. The introduction of one or two fluorine in the sidechain resulted in a slight decrease or similar antitubercular activity [40]. Subsequently, by increasing the hydrophilicity of the sidechain and reducing the linker to two-carbon in 7-position, the increase in activity of compounds 19 and 20 against Mtb LeuRS (19-GSK656, IC50, 0.20 μM; 20, 0.12 μM) [40] was achieved. Existing equilibrium between an open and a closed form of 19–21 and 20–22 of the benzoxaborole pharmacophore has a dependency on solvent and environment [41]. In addition, the ring-fused compounds of 23 and 24 exhibited enhanced anti-tubercular activity against Mtb H37Rv with the MIC of 0.08 μM and 0.03 μM, respectively, and potent Mtb LeuRS activity of IC50 of 0.046 μM and 0.12 μM for 23 and 24, respectively [41]. Compounds 19 and 23 exhibited low clearance and excellent exposure in drug metabolism and pharmacokinetics studies. The typical Mtb LeuRS inhibitor shows low molecular weight, low polar surface area (PSA), and clogD7.4 value similar to frontline Mtb drugs of isoniazid, pyrazinamide, and ethambutol [40].
To evaluate the ability of these Mtb LeuRS inhibitors for tuberculosis, treatment tests were conducted in vivo using an animal model. Compound 19 showed the best efficacy with an ED99 (efficacious dose that gives a two log colony-forming units (CFU) reduction compared to the untreated control) of 0.4 mg/kg among the evaluated compounds. For the best profile, with excellent in vivo efficacy at low doses in acute and chronic mouse TB infection models, compound 19 has been progressed to clinical development for the treatment of tuberculosis, the first time in Human (FTIH) safety and pharmacokinetics (PK) study of GSK3036656 in Healthy Subjects [42].
Patel et al. identified a novel 6-benzyl ether benzoxaborole 25 with potent activity against Mtb in vitro (MIC 2 μM), which was active against intracellular bacteria (50% inhibitory concentration IC50 3.6 μM) with no cytotoxicity; thus, the profile of this compound is also encouraging for future development [43]. Meanwhile, a series of novel 7-phenyl benzoxaboroles were also investigated, where compounds 26–29 showed reasonable activity against Mtb in vitro (5.1–80 μM) with lower MIC99 (the concentration required to inhibit growth by 99%) (5–12.5 μM) [44]. These compounds may target NADH dehydrogenase (Ndh) rather than LeuRS [44]. Ndh is an essential oxidoreductase, which catalyzes the electron transfer process from NADH to menoquinone as part of the electron transport chain [45], and mutations in Ndh, found in clinical isolates, have shown resistance to isoniazid [46]. Further studies revealed that these processes correspond to residues involved in the quinone binding pocket [47]. This series of compounds shows potential for further development and to target validation work. In addition, dimeric benzoboroxoles were reported recently, and they were found to possess excellent selectivity and activity for mycobacteria, including the Mtb pathogen, and were capable of complexing to Mtb glycans without resistance [48].
2.2. Peptidyl Boronates/Boronic Acids
Boronates may interact with a target protein through covalent bonding with nucleophilic entities (such as hydroxyl and amine groups of enzymes, Figure 1B) to form a stable bond with the enzymes, thereby leading to their reversible inhibition. The boronic acid species can be incorporated with a peptide to form the corresponding peptidyl boronate/boronic acid, which may exhibit various biological activities [49,50]. Bortezomib (Takeda Pharmaceutical) (1, Figure 1C), trade name Velcade, is a dipeptide boronic acid and is the first human proteasome (H. proteasoma) inhibitor approved by the U.S. FDA for the treatment of multiple myeloma [51]. The X-ray crystal structure of the proteasome in a complex with bortezomib displayed a covalent bond formation between the boronic acid moiety of 1 and the hydroxyl group of Thr1 at the chymotrypsin-like active site of the 20S proteasome, leading to enzyme dysfunction and apoptosis in cancer cells [52,53] (H. proteasome IC50 0.005 µM). However, bortezomib presented major drawbacks, such as high costs and poor pharmacokinetics with significant side effects (peripheral neuropathy, neutropenia, and cytopenia) despite its use to treat many cancers successfully [54].
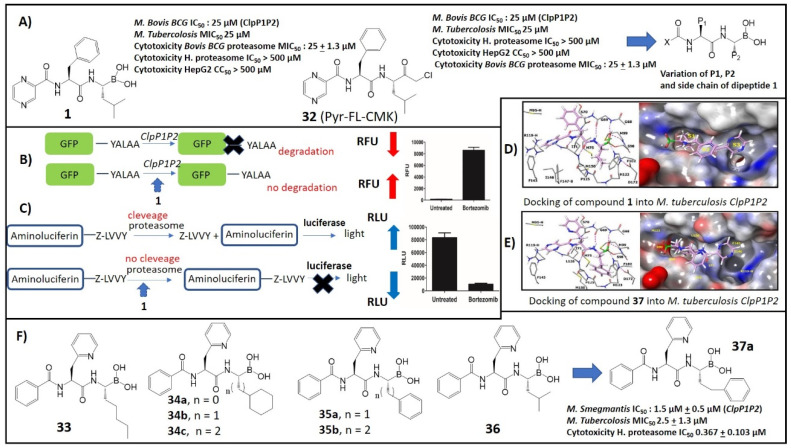
Caseinolytic proteases (ClpP) are serine proteases found in a wide range of bacteria, and they have the ability to remove the aborted translation products [55]. The tmRNA trans-translation system, a bacterial rescue system that frees ribosomes stuck during protein synthesis, tags partially synthesized proteins with a caseinolytic-protease-specific (SsrA) degradation peptide. The SsrA-tagged proteins are recognized by the ClpP and degraded [56,57]. Mycobacteria, including Mtb and Mycobacterium smegmatis, encode two ClpP homologs, clpP1 and clpP2, in a single operon which associate together to form a single proteolytic complex, referred to as ClpP1P2. The caseinolytic protease complex is composed of catalytic protease subunits (ClpP) and regulatory subunits (ATPases). Both proteins are required for viability in vitro and during infection, and depletion of either protein results in the rapid death of the bacteria [58]. Genetic studies also suggest ClpP may serve as an ideal target for antimycobacterial therapy because of the synergistic nature of ClpP1P2 protease depletion with mistranslation-inducing aminoglycosides that are important second-line drugs for Mtb [58]. Compound 1 was identified as a whole-cell-active ClpP1P2 protease inhibitor in mycobacteria and a new lead compound for TB (M. Bovis IC50 ClpP1P2, 1.6 ± 0.5 µM, M. Smegmatis MIC50 6 µM) through the mechanism-based whole-cell screening method from a library of over 500000 compounds (Figure 4A) [59]. To measure the intracellular ClpP1P2 inhibition, Dick et al. engineered an M. smegmatis through screening that allows the detection of inhibitors of intracellular ClpP1P2 activity via the accumulation of SsrA-tagged green fluorescent protein (GFP) (Figure 4B). In normal conditions, the ClpP1P2 complex recognizes the SsrA- (YALAA) tagged with GFP (GFP-SsrA) and degrades the proteins, resulting in low basal fluorescence. In the presence of an inhibitor (Bortezomib, 1), ClpP1P2 binds to the catalytic sites of the protease and prevents the degradation of the GFP-SsrA proteins, resulting in its accumulation and a gain of fluorescence signal (Figure 4B) [59]. On the other hand, mammalian proteasome intracellular inhibition was measured using the whole-cell target-based proteasome-Glo assay, as shown in Figure 4C. This assay is based on a proteasome-specific cleavage tag (Z-LLVY) that fuses to an aminoluciferin molecule. In normal conditions, the proteasome cleaves the LLVY tag, allowing the luciferase to oxidize the aminoluciferin generating luminescence. In the presence of a proteasome inhibitor, the LLVY cleavage does not occur. The tagged aminoluciferin cannot be used by the luciferase enzyme, which is preventing the subsequent emission of luminescence [60].
Figure 4.
(A) Structures and antifungal activity of selective Mycobacterial ClpP1P2 inhibitors 1 (Bortezomib) and 32. (A series of dipeptidyl boronates with variation at the P1, P2, and X side-chains were synthesized); (B) ClpP1P2 inhibition assay; (C) Proteasome inhibition assay; (D) Docking of 1 into Mtb ClpP1P2; (E) Docking of 37a into Mtb ClpP1P2; (F) Structures and antifungal activity of selective Mycobacterial ClpP1P2 inhibitors 33–37a (Adapted from [59,62]).
Chloromethyl ketones (CMKs) comprise a distinct class of covalent irreversible serine protease inhibitors [61]. The function of this class of peptide is mechanistically similar to that of boronic acids. Compound 32 (pyrazine-phenylalanine-leucine-chloromethylketone), an analog of 1 containing a chloromethyl ketone (CMK) instead of the boronic acid, was synthesized and its potencies against the bacteria and human enzymes were determined [61]. Compound 32 retained its activity against mycobacterial ClpP1P2 (IC50: 25 µM), against bactericidal Mtb (IC50: 25 µM) and was active against the mycobacterial proteasome (MIC50: 25 ± 1.3 µM), but was found to be devoid of activity against the mammalian human proteasome (IC50: >500 µM vs. 1 IC50: 0.005 µM) [61]. The CMK analog was not toxic to HepG2 cells at a concentration of up to 500 μM, while bortezomib displayed a cytotoxicity CC50 of 250 µM [61]. These chloromethyl compounds similarly inhibited both ClpP1P2 and the proteasome in the bacteria while leaving the human proteasome untouched. These results suggest that the selectivity over the human proteasome is achievable [61]. Based on these results, a series of dipeptidyl boronate derivatives of 1, with variation at the P1, P2, and X sidechains, were synthesized with a goal to identify compounds which inhibit bacterial ClpP1P2 in a bacterial cell and have reduced potency against the human proteasome compared to bortezomib (Figure 4A) [62]. Replacing the iso-butyl group in P1 of 1 with a less hindered straight-chain n-pentyl (compound 33, Figure 4F) increased the activity against Mtb twofold, whereas it decreased the potential in the proteasome assay by 6-fold (IC50: 0.03 µM) [62]. Aromatic derivatives of 35 showed 10–14-fold-lower potency for the proteasome compared to 1 [62]. Subsequent studies showed that a bulky group (benzyl and phenyl) in position X could increase the ClpP1P2 inhibitory activity without a reduction in proteasome activity. Different bulky heterocyclic groups were also screened, and among them compound 36 with the 3-pyridyl group provided an interesting result of 6-fold-lower potency for the proteasome compared to 1 with retention of ClpP1P2 inhibitory activity [62]. This series of changes of X offers options for subsequent P1–P2–X combinations for the future phase of SAR exploration.
Docking studies suggested a larger P1 ligand could be accommodated in the P1 pocket of the ClpP1P2 but less well tolerated in the P1 pocket of the human proteasome (Figure 4D). The docking of 37a to the binding site of ClpP1P2 indicates that the hydrophobic S1 residues Ile71, Met75, Met99, Phe102, and Pro125 interact with P1 (phenethyl group). Hydrogen bonds are also formed between the P2 amine and the backbone carbonyl of Leu126 and between the carbonyl of the N-terminal and the backbone amine of Ile71 (Figure 4E) [62]. In medicinal chemistry, the “drug likeness” of this selected compound was commonly investigated and predicted from its pharmacokinetic properties. Physicochemical properties such as molecular weight, numbers of hydrogen bond donors and acceptors and lipophilicity (LogP) were examined according to Lipinski’s rule of five [63]. Compound 37a was selected for further profiling in vitro ADME assays (absorption, distribution, metabolism, and excretion). It had favorable in vitro ADME properties: plasma protein binding and human liver microsome stability was moderate, clearance in mouse microsomes was high (8min), and the inhibition of cytochrome P450 enzymes was not detected at the highest concentration tested. The Oral/i.v. pharmacokinetics of 37a indicated moderate clearance and low bioavailability [62,64]. Therefore, ClpP1P2 inhibitors are a possible new strategy for the management of drug-resistant M. Tubercolosis.
2.3. Other Small Compounds of Boron (Diazoborines, Antibiotic)
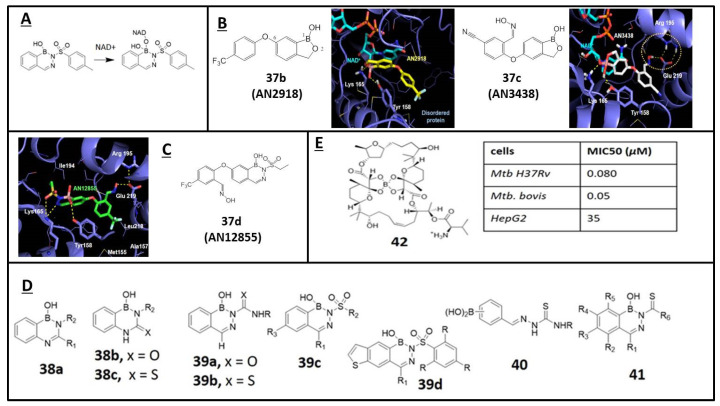
Diazaborines are a family of boron-containing compounds, in which the boron atom is stabilized in the form of an aromatic boron-based heterocycle. The antibacterial activities of 1,2-dihydro-l-hydroxy-2-(organosulfonyl)arenol-[d]-[1,2,3]-diazaborines are well documented in the literature [65]. It has been proposed that the mechanism of action of diazaborines in E. coli is by the complexation of nicotinamide adenine dinucleotide (NAD+) and the inhibition of enoyl-reductase (ENR) [66]. Similar to the benzoxaboroles such as 37b (AN2918) and 37c (AN3418), diazaborine inhibitors of ENR were found to form a covalent B–O bond with the OH group at C (2′) of the NAD cofactors ribose unit (Figure 5A,B) [67,68]. Mycobacteria have a similar enzyme with enoyl-reductase, InhA (Enoyl-[acyl-carrier-protein] reductase [NADH]), which is required for mycolic acid biosynthesis [69]. Recently, diazoborine 37d (AN12855), which exhibited in vitro bactericidal activity against replicating bacteria, was revealed to inhibit the substrate-binding site of InhA in a novel cofactor-independent manner (IC50: InhA 0.03 µM, Figure 5C) [68].
Figure 5.
(A) Formation of a covalent B–O bond of diazaborines with the OH group at C (2′) of the NAD cofactors ribose unit of enzyme ENR; (B) Structure of oxaborole 37b (AN2918) and 37c (AN3418) and their complex crystal structure with Mtb InhA; (C) Structure of diazoborine 37d (AN12855) and its complex crystal structure with Mtb InhA; (D) Structures and antifungal activity of 2,3,1-benzodiazaborines 38–41; (E) Structures and cytotoxicity activities of Boromycin 42 (Adapted from [68,70,71,72,73]).
Martin et al. first reported the synthesis of 2,4,1-benzodiazaborine compounds 38a–c (R1= -pyrazinyl/R2 -H, -nBu, -pyridyl), showing potent inhibitory activity against M. tuberculosis (Figure 5D) [70]. Subsequently, a set of 2-acylated 2,3,1-benzodiazaborines 39a–d was synthesized, characterized, and tested with Mycobacterium smegmatis (Figure 5D) [71]. In addition, 2-formylphenyl boronic acids 40 (R= H, allyl, Ph) and their derivatives of 41 were also reported as potential antifungal agents, and their activity was examined against four fungi (Aspergillus niger, Aspergillus flavus, Candida albicans, and Saccharomyces cerevisiae) using Amphotericin B as a control and showed appreciable activity [72,73]. Boromycin 42 is a boron-containing polyether macrolide antibiotic isolated from Streptomyces antibioticus. It is a potent inhibitor of mycobacterial growth (MIC50: 80 nM) with strong bactericidal activity and low cytotoxicity vs. HepG2. It acts as an ionophore and causes the collapse of the potassium gradient across the bacillus’ membrane (Figure 5E) [74].
3. Malaria
Malaria, a parasitic infection by the Plasmodium genera, is spread through the bites of infected mosquitoes Anophele. It was responsible for an estimated 229 million clinical cases and 409,000 deaths worldwide in 2019, mostly among children under the age of five years [75]. Malaria is transmitted by parasites of the Plasmodium genus with five species known to infect humans: P. falciparum, P. malariae, P. vivax, P. ovale and P. knowlesi, with infections by P. falciparum (Pf.) and P. vivax being the most virulent [76]. Human malaria infection is initiated when a female anopheles mosquito deposits “sporozoites” during a blood meal. These sporozoites migrate to the liver where they undergo further development into schizonts, which produce “merozoites” that enter into the systemic circulation where they infect red blood cells and cause the typical symptoms of malaria. Some merozoites in these cells may develop into an asexual form called “trophozoites”, and in some cases into sexual forms of the parasite, called “gametocytes”, that circulate into the bloodstream. When a mosquito bites an infected human, it ingests the gametocytes, which develop further into mature sex cells called “gametes”. In the mosquito’s stomach, the male microgametes penetrate the female macrogametes, generating “zygotes”. The zygotes invade the midgut wall of the mosquito where they develop into “oocysts”. The oocysts grow, rupture, and release “sporozoites” which enter mosquito’s salivary glands. The inoculation of the sporozoites into a new human host will start a new malaria life cycle [77]. Chloroquine (CQ) was one of the most widely used antimalarial drugs, which has been now substituted by artemisinin (ART) and its synthetic derivatives [78]. The successful exploitation of semisynthetic ART derivatives was a major breakthrough in malaria chemotherapy because of their profound and rapid therapeutic response against malaria parasites. The WHO recommends that deadly species P. falciparum should be treated with artemisinin-based combination therapies (ACT), in which the ART-based component is combined with a second, longer-acting partner drug. However, reports of decreased efficacy, reduced parasite clearance time in the case of ACT treatment and widespread resistance by Plasmodium parasites [79,80] suggest the need for a new search for novel pharmaceutical interventions for malaria [81].
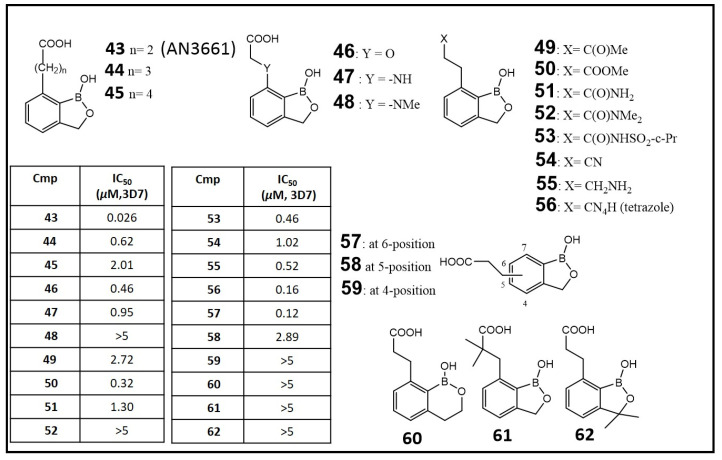
Early observation of antifungal, antibacterial and anti-inflammatory activities of benzoxaboroles led to the discovery of their potential for therapy of protozoan disease such as malaria, human African trypanosomiasis (HAT) and Chagas disease [81]. After screening in a whole cell assay against the malaria parasite P. falciparum of a boron-containing compound collection, Zhang et al. reported some potent hits (Figure 6), including 7-(2-carboxyethyl)-1,3-dihydro-1-hydroxy-2,1-benzoxaborole (43, AN3661, IC50: 0.026 μM). A series of analogs of 43 were designed to assess the structural features required for potent antimalarial activity, including the length of the sidechain on the oxaborole nucleus (44, 45), the sidechain functional groups (46–56), the attaching positions of the sidechain (57–59), and modifications to the benzoxaborole scaffold (60–62) (Figure 6) [82,83]. Further structural modification, such as the introduction of fluoro, phosphonic and hydroxamic groups, was found to decrease the activity potency dramatically; it was also that the removal of the boron atom from the five-membered oxaborole ring reduced the antimalarial activity [84].
Figure 6.
Structures and antimalarial activity (IC50) of benzoxaborales (43–62) against the malaria parasite Plasmodium falciparum CQ-sensitive 3D7 strain. (Adapted from [82]).
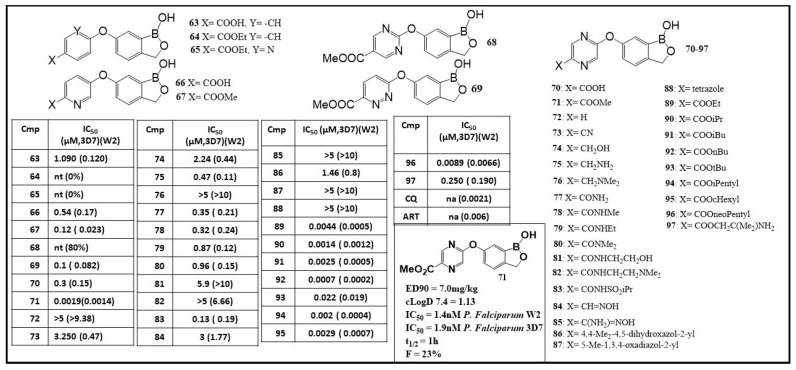
A new scaffold of 6-(4-carboxyphenoxy)-1,3-dihydro-1-hydroxy-2,1-benzoxaborole, 63 (shown in Figure 7), was identified in 2015 with a potent activity of IC50 of 0.120 μM against P. falciparum [85]. Further studies of structure–activity relationship (SARs) were performed by varying the 6-aryloxy group (64–71), substituent modification on the pyrazine ring (72–88) and exploring the effect of side-chain ester group (89–97) (Figure 7). To examine the effect of the left-side aromatic moiety on antimalarial activity, compounds 64–71 were designed. If the nitrogen was in an ortho-position to oxygen as in 65, the incorporation of a nitrogen atom did not improve the activity, whereas nitrogen located at the meta-position to oxygen would increase the activity of 66 and 67 [85]. A pyrazine ring-embedded compound (71) showed extremely high potency both for Pf W2 strain and Pf 3D7 (IC50: 0.0014 μM for Pf W2; IC50: 0.0019 μM for Pf 3D7) [85]. With the identification of the pyrazine ring, compounds 72–88 were synthesized to investigate the effects of the substituent group in the ring. None of the compounds showed antimalarial activity better that 71, and that would indicate the presence of the carboxylic ester as a crucial functionality. Other ester compounds (89–97) were designed and synthesized to further explore the effects of different esters (Figure 7). Compound 92 containing n-butyl ester showed outstanding potency with an IC50 value of 0.0002 μM for Pf W2 and 0.0007 μM for Pf 3D7, respectively [85,86]. Compound 71 demonstrated excellent efficacy in vivo against P. berghei in infected mice (ED90 7.0 mg/kg). Nevertheless, the metabolic instability and less favored PK parameters of 71, such as relatively short half-life and low bioavailability, warrant its further optimization (Figure 7, panel A) [85,86].
Figure 7.
Structures and antimalarial activity (IC50) of benzoxaboroles (63–97) against the malaria parasite Plasmodium falciparum CQ-sensitive 3D7 and CQ-resistant W2 strains. (Adapted from [85]).
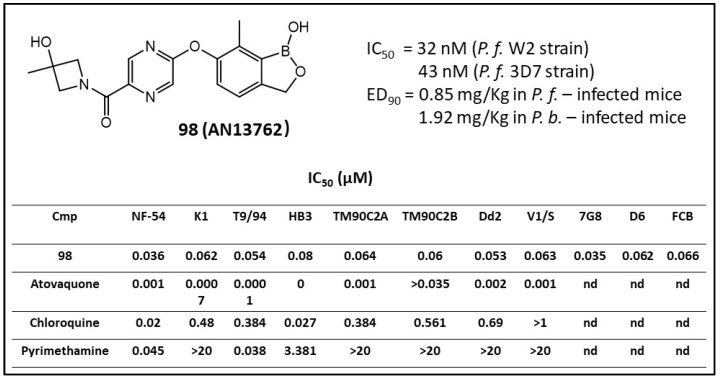
To optimize the potency, stability and PK profile of such benzoxaborole derivatives, various carboxamide functional groups were incorporated. Analogues to different 1′-monoalkyl substituents in the amide sidechain had potencies ranging from 0.031 to 1.99 µM against Pf CQ-sensitive strain 3D7 and showed that the (R-) enantiomers were generally little more active than the (S-) isomers [86]. Among the screening compounds, compound 6-(2-((3-hydroxy-3-methylazetidin-1-yl) carbonyl) pyrazinyl-5-oxy)-1,3-dihydro-1-hydroxy-7-methyl-2,1-benzoxaborole (98, AN13762) (Figure 8) was chosen as a lead compound, which showed an ED90 value of 1.9 mg/kg. The result of the P. falciparum-infected mouse model experiment demonstrated that the in vivo parasite clearance profile of 98 was rapid and similar to that of artesunate (water-soluble injectable derivative of ART) and chloroquine, two well-known fast parasite-killing antimalarial medicines [86]. Compound 98 (AN13762) was subjected to potency evaluation against other resistant P. falciparum strains, in vivo parasite reduction rate evaluation (or number of parasites the compound could kill in a parasite life cycle, PRR), and for preliminary genotoxicity studies. An in vitro PRR assay against P. falciparum was used to compare the parasitic killing rates at different concentrations. The results indicated that the antiparasitic rate of action of 98 was fast and similar to those for ART and chloroquine. Further, 98 was also examined against an additional eleven P. falciparum resistant strains which demonstrated high activity with the IC50 value in the range of 0.036−0.080 µM, indicating no cross-resistance (Figure 8). Safety studies demonstrated that it was not mutagenic and clastogenic in both the in vitro and in vivo models [86]. Therefore, 98 was further investigated for the development of preclinical studies in humans beginning in 2019 (MMV-Supported Projects. https://www.mmv.org/research-development, accessed on 18 January 2021).
Figure 8.
In vitro activities of 98 against multiple P. falciparum parasite strains (IC50) (Adapted from [86]).
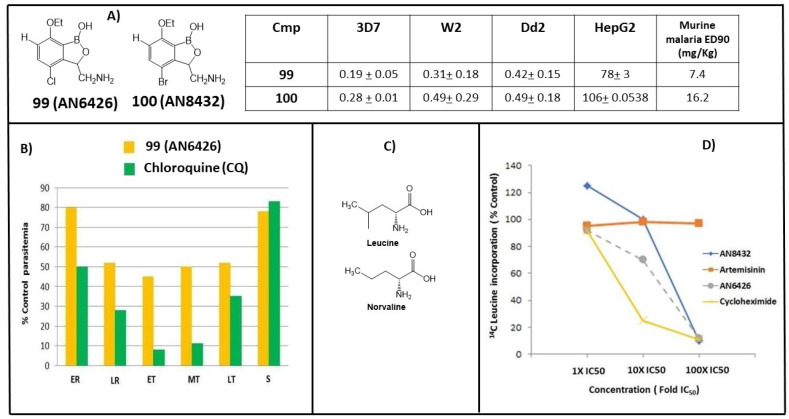
Compound 98 showed no cross-resistance property and that indicated a possible novel action mechanism or drug resistance of benzoxaboroles that is different from those of CQ and pyrimethamine. The highly electrophilic nature of the boron component of these compounds could lead to interactions with a variety of protein targets via reversible covalent bonds (Figure 1B). The benzoxaboroles 2, 10 (AN3018), AN3365 and AN3664/ZCL039 (Figure 2C) inhibited bacterial LeuRS in an action mechanism [87,88]. In the course of searching for new antimalarial drugs, a benzoxaborole library of LeuRS inhibitors was screened for potency against cultured multidrug-resistant W2 P. falciparum strains and the antimalarial activity was investigated [89]. The two most active 3-aminomethylbenzoxaboroles, 99 (AN6426) and 100 (AN8432) (Figure 9A), were selected and extensively examined. The compounds demonstrated the murine malaria ED90 values of 7.4 mg/kg and 16.2 mg/kg for 99 and 100, respectively, in vivo to P. berghei-infected mice. Subsequently, 99 and CQ were investigated in different stages of parasites, and inhibition of parasite development was observed across the life cycle of plasmodium, particularly against trophozoites (Figure 9B) [89]. This inhibition happened only with exogenous norvaline (unnatural amino acid analogue of leucine that is charged to tRNA by LeuRS enzymes), rather than with AN6426-resistant parasites. The results are consistent with a loss of LeuRS editing (Figure 9C). Biochemical studies showed that 99 and 100 caused a dose-dependent inhibition with the incorporation of [14C] leucine, indicative of a block in wild-type protein synthesis (using artemisinin as a negative control and, as a positive control, cycloheximide, protein synthesis inhibitor) (Figure 9D) [88,89].
Figure 9.
(A) In vitro (3D7 and W2/D2d P. falciparum strains) and in vivo (P. bergei strains) antimalarial activity (IC50, µM) of 99 (AN6426) and 100 (AN8432); (B) Stage specificity of 99 and CQ; (C) Chemical structures of Leucine and analogue Norvaline; (D) Effects of benzoxaboroles 99 and 100 and controls ART and Cycloheximide on [14C] leucine incorporation by wild-type Dd2 strain P. falciparum. (Adapted from [88]).
During the screening process, 3-(1-hydroxy-1,3-dihydro-2,1-benzoxaborol-7-yl)-propanoic acid was identified as a potent antimalarial agent against P. falciparum asexual blood stage parasites known to be resistant to standard antimalarial drugs [90]. The compound was highly effective when administered orally to treat P. berghei (ED90: 0.34 mg/kg) and P. falciparum (ED90: 0.57 mg/kg) infections in mice, with minimal cytotoxicity to mammalian cell lines. Its inhibitory effects were greatest in early to middle trophozoite-stage parasites [90]. Enzyme CPSF-73 is a metallo-b-lactamase containing two zinc ions essential in the active site [91]. The PfCPSF3 is a Plasmodium homologue of mammalian CPSF-73. Docking calculation of the compound on the PfCPSF3 active site revealed that its terminal carboxylate group, occupying an adjacent phosphate-binding site opposite to R290 and Y252, forms a salt bridge and a hydrogen bond, respectively [90]. The negatively charged tetrahedral oxaborole group was placed at the phosphate position at the cleavage site and it interacted with the two catalytic zinc ions. In these models, the identified PfCPSF3 resistance mutations (T406I, Y408S, T409A and D470N) were found on the PfCPSF3 active site of amino acids interacting with AN3661 [90].
4. Neglected Tropical Diseases (NTD)
4.1. Trypanosomiasis
Human African trypanosomiasis (also known as African sleeping sickness or HAT), a Neglected Tropical Disease (NTD) that occurs in sub-Saharan Africa, is transmitted to humans through the bite of different species of tsetse fly (Glossina spp.). It presents a major threat to the health of more than 57 million people in 36 countries in sub-Saharan Africa [92]. During a blood meal on the mammalian host, an infected tsetse fly injects “trypomastigotes” (a parasitic flagellate protozoa) into skin tissue [93]. The parasites enter the lymphatic system, pass into the bloodstream (stage I, hemolymphatic system) and then transform into bloodstream trypomastigotes, which are carried to other sites (stage II, CNS, central nervous system, spinal fluid). The disease is caused by unicellular Trypanosoma brucei gambiense (T. b. gambiense), which is endemic in western and central Africa, or Trypanosoma brucei rhodesiense (T. b. rhodesiense), which is found in eastern and southern Africa [93]. The currently available drugs for the treatments for early-stage infection (stage I) are pentamidine and suramin, while melarsoprol and eflornithine are for late-stage infection (stage II or CNS). All these drugs share the same problems of high cost and toxicity with low efficacy in the late stage and potential development of resistance, and they are not orally bioavailable. Thus, there is an urgent need to develop bioavailable oral treatment with improved efficacy and low toxicity at an affordable cost for the treatment of HAT [92,93].
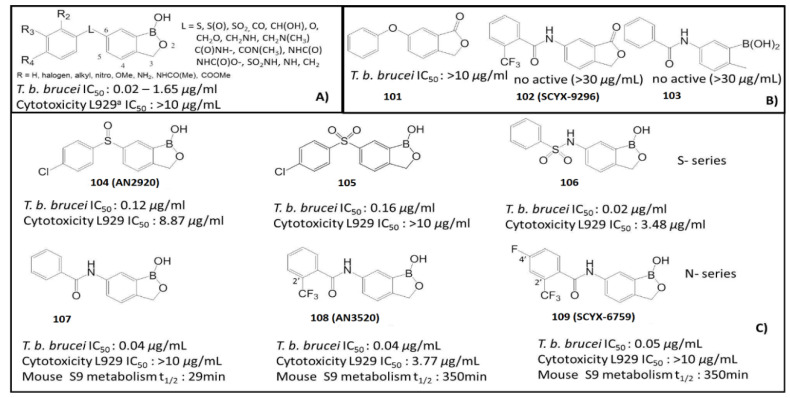
In 2010, the UCSF Sandler Centre of Drug Discovery, in collaboration with Anacor Pharmaceuticals, identified several compounds through an antitrypanosomal screening of 400 compounds, leading to the discovery of drugs with high potency to inhibit T. b. brucei, as shown in Figure 10. Preliminary results of the structure–activity relationships (SAR) suggested that benzoxaboroles containing a substituent at C (6) of the heterocyclic ring system were particularly essential (Figure 10A) [94]. Thus, the oxaborole functionality was crucial for the observed antitrypanosomal activity, as demonstrated by low activity (IC50 > 10 μg/mL) or loss of activity upon removal of the oxaborole ring or substitution with carbon (101–109) (Figure 10). The length between the hydrogen bond acceptor O and the benzoxaborole C(6) of the linkage group “L” had a significant effect on the antitrypanosomal activity (i.e., in sulfonamide, O-C(6) distance 3.52 Å, IC50 0.02 µg/mL vs. sulfoxide, O-C(6) distance 2.38 Å, IC50 0.17 µg/mL). Compounds with amide linkers showed high potency. Accordingly, the most potent compounds among the series were benzoxaboroles with a sulfonamide linker (106) and amide linker (107) that showed an improvement in antitrypanosomal activity with an IC50 of 0.02 and 0.04 µg/mL, respectively, to inhibit T. b. brucei (Figure 10C) [94]. The in vivo assessments using the murine model of blood stage (I) T. b. brucei infection showed that the sulfone linker in 105 was more efficacious, with complete cure observed at 20 mg/kg. The sulfonamide linker in 106 exhibited modest in vivo activity with a serious cytotoxicity of 3.48 µg/µL) [95]. By the modification of an amide linked compound, new leads, N-(1-hydroxy-1,3dihydrobenzo[c] [1,2] oxaborol-6-yl)-2-trifluoromethylbenzamide (108, AN3520) and 4-fluoro-N-(1-hydroxy-1,3-dihydrobenzo[c] [1,2] oxaborol-6-yl)-2-trifluoromethylbenzamide (109, SCYX-6759), were identified (Figure 10C) [95]. These two compounds exhibited high permeability, in vitro metabolic stability (Mouse S9 metabolism t1/2 > 350 min), and rapid time-dependent trypanocidal activity against T. b. brucei. Pharmacokinetic analysis demonstrated that 108 and 109 were orally bioavailable in multiple species and were able to cross the blood–brain barrier (BBB) at sufficient levels to cure stage II of the HAT disease in mice, with no evidence of interaction with the P-glycoprotein transporter [96]. These oxaborole carboxamides cured stage I (hemolymphatic) trypanosomiasis infection in mice when administered orally at 2.5 to 10 mg/kg of body weight for 4 consecutive days. Metabolism and pharmacokinetic studies in several species, including nonhuman primates, demonstrated that both 108 and 109 were low-clearance compounds [94,95,96].
Figure 10.
(A) Principal linker L in position C(6) of benzoxaboroles; (B) Structures and antitrypanosomal activity of no boron analogues 101–103; (C) Structures, antitrypanosomal activity, cytotoxicity and biological half-life t1/2 of benzoxaborole derivatives S-series 104–106 and N-series 107–109 (Adapted from [94]).
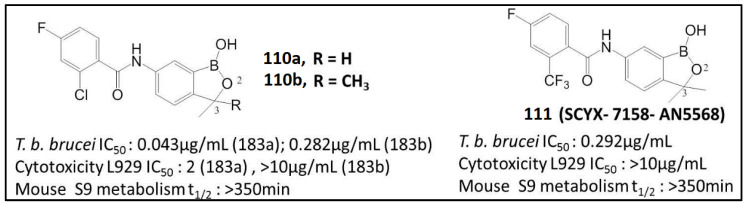
Sulfonamide 106 was further modified using various linkers between the heterocyclic core and pendant aryl group to show reasonable potency in the whole-cell T. b. brucei assay with low cytotoxicity (IC50 > 10 µg/mL for mouse lung fibroblast cells (L929)) [97]. The introduction of a methyl group (110a) at C(3) of the benzoxaborole ring had little effect on the trypanocidal potency but caused a significant increase in cytotoxicity (110a vs. 110b), while C(3)-dimethyl analogs (110b and 111) retained trypanocidal activity but were not cytotoxic (Figure 11) [97]. Compound SCYX-7158 (111) exhibited enhanced activity against representative strains of T. b. brucei, including T. b. rhodesiense and T. b. gambiense strains (from 0.07 μg/mL to 0.37 μg/mL), following the incubation of the parasite strains with the compound for 72 h [98]. The in vivo activity of these oxaboroles was assessed using the mouse model of acute and chronic HAT. The SCYX-7158 exhibited good permeability across the blood–brain barrier and achieved in measurable levels after both intravenous and oral doses. Phase I assessed the safety, tolerability, pharmacokinetics and pharmacodynamics of SCYX-7158 by applying a single oral ascending dose in 128 healthy human volunteers of sub-Saharan origin. It allowed the therapeutic dose administered at 960 mg once as three tablets, with a favorable safety profile. As the drug has a long half-life (>300 min), the study was extended to 210 days to ensure safety monitoring of the healthy volunteers [99]. Based on the results of this study, DNDi (Drugs for Neglected Diseases Initiative) and partners proceeded to Phase II/III—efficacy and safety study of SCYX-7158 as a single dose oral treatment of patients with HAT [100].
Figure 11.
Structures, antitrypanosomal activity, cytotoxicity and biological half-life t1/2 of benzoxaboroles 110 and 111 (Adapted from [98]).
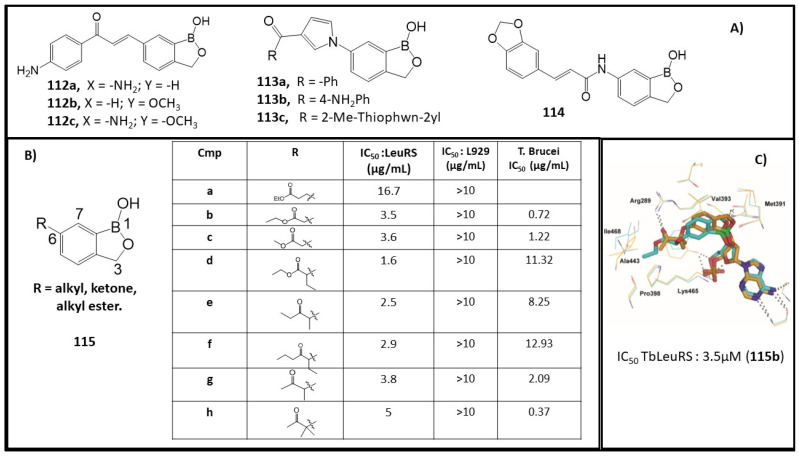
Chalcones have attracted considerable scientific attention and continue to be a versatile scaffold in anticancer and antiprotozoal research. Previously, chalcone-type compounds were found to inhibit the growth of T. b. brucei and Trypanosoma cruzi parasites [101]. A novel class of chalcone–benzoxaborole hybrid molecules was synthesized and evaluated as an antitrypanosomal agent. The 4-NH2 derivative 112a and 3-OMe derivative 112b (Figure 12A) were found to have excellent potency against T. b. brucei (112a, IC50: 0.024 µg/μM; 112b, IC50: 0.022 µg/μM) and good cytotoxicity (L929 cells, IC50 > 10 μg/mL). The synergistic 4-NH2-3-OMe compound 112c presented a high toxicity (L929 cells, IC50: 1.45 μg/mL) [102]. The 6-pyrrolobenzoxaboroles, 113, represent a new class of potent antitrypanosomal agents. These compounds showed an antiparasitic activity ranging from 0.03 µg/mL to 4.02 µg/mL [103]. Three of the leading compounds (113a–c) demonstrated high in vitro activity against T. b. brucei (IC50: 0.09 µg/mL for 113a; 0.03µg/mL for 113b; 0.07 µg/mL for 113c) and good cytotoxicity (L929 cells, IC50 > 10 μg/mL for 113a and 113c). They also showed good possibility to cure the parasitic infection in a murine acute infection model with complete clearance of the parasites in the blood (Figure 12B) [103]. Meanwhile, a set of cinnamoyl–oxaborole amides were also synthesized and screened against nagana T. b. brucei for antitrypanosomal activity. Compound 114 emerged as a new hit with an in vitro IC50 value of 0.086µM against T. b. brucei without inhibitory cytotoxicity against HeLa cell lines (Figure 12A) [104].
Figure 12.
(A) Structures of chalcone–benzoxaborole 112, pyrrolobenzoxaborole 113 and cinnamoyl–oxaborole 114; (B) Structures, antitrypanosomal activity and cytotoxicity of benzoxaboroles 115a–h; (C) Binding of ester 115b (ligand in cyan sticks and binding site residues in cyan lines) in the editing pocket of TbbLeuRS (Adapted from [87,104,105]).
As discussed before, compound 2 (Figure 1), which is under clinical investigation, was indicated as an antifungal agent by inactivating fungal LeuRS [32]. Encouraged by the inhibitory activity of such compounds, C(6)-ester group-functionalized 115a and 115b were synthesized, while 115b showed a 4-fold improvement in activity (TbbLeuRS IC50: 3.5 μM) compared to 188a (TbbLeuRS IC50: 16.7 μM) (Figure 12B) [105]. Compounds 115c–i were also screened as an effort to improve the stability of the leading ester compounds in vivo while retaining their activity. The addition of methyl or ethyl substituents in the α-position to ketone resulted in a significant enhancement of activity, as demonstrated by compounds 115f–I (TbbLeuRS IC50 2.5, 2.9 and 3.8 μM, respectively) (Figure 12B). The docking model of compound 115b showed the formation of a hydrogen bond between its carbonyl and Arg289. The pocket is rather small and hydrophobic, lined by nonpolar amino acid residues including Pro398, Ala443, Ile468, and Ala464, and is a good fit with the terminal ethyl group of the compound 115b. The docking model of compound 115b also revealed the existence of space near the carbon of the ester (Figure 12C) [105]. These TbbLeuRS inhibitors showed good potency against the bloodstream form of T. b. brucei parasites (T. b. brucei IC50: 0.37–12.93 μM). Although these substituted ketones exhibited similar enzyme inhibitory activity, the dimethyl ketone derivative, 115h, showed higher potency (T. b. brucei IC50: 0.37 μM) than its methyl analogue [105].
4.2. Leishmaniasis
Leishmaniasis is a vector-borne parasitic disease caused by at least twenty species of the genus Leishmania, with three main clinical forms of visceral leishmaniasis (VL), cutaneous leishmaniasis (CL) and mucocutaneous leishmaniasis [106]. This disease is responsible for 700,000 to 1 million new infection cases annually. When an infected female sand fly bites the skin of a person or animal, the Leishmania parasites promastigotes (protozoan parasites) are injected into a new host. Once on the skin, promastigotes are ingested by phagocytic cells and the parasites differentiate into obligate intracellular amastigotes. These parasites replicate and invade other sites of the body. The cycle continues until a sand fly bites the infected individual, taking up some of the amastigotes during the process [107]. The absence of effective vaccines gives way to treatment by chemotherapy using drugs such as pentavalent antimonials and amphotericin B as primary control of the disease [108]. However, these drugs require parenteral administration. They are nephrotoxic and an increasing drug resistance in visceral leishmaniasis has been identified [109]. The efficacy of the first-line oral drug, miltefosine, has declined rapidly over the past decades due to treatment failure, which results in relapses of the disease [110]. The WHO lists leishmaniasis as one of the NTDs and advocates an urgent need for new, efficient, safe, and affordable drugs for the treatment [111].
In a new drug screening process, leucyl-tRNA synthetase from L. donovani (LdLRS) was selected as a potential drug target for Leishmania. This enzyme plays an essential role in the viability of this pathogenic organism and appears to be indispensable for its survival in vitro [112]. Compound 2 (Figure 1) exhibited anti-leishmanial activity against both promastigote and amastigote stages, in vitro, as well as in vivo in BALB/c mice, as shown in Figure 13A. Moreover, 2 was effective in inhibiting the aminoacylation activity of the recombinant LdLRS (IC50: 0.83 ± 0.2 μM), with low toxicity to mammalian cells [112]. Recently, protozoan carbonic anhydrases (CAs) were explored as new targets for drug development for bacteria, fungi and protozoa [113,114]. A type of 6-substituted urea/thiourea benzoxaboroles was tested against CAs from the two pathogenic protozoans (L. donovani and T. cruzi) [115]. Acetazolamide, a clinically used sulfonamide inhibitor, and Tavaborole 2, a commercial benzoxaborole used as topical antifungal medication, were used as standard control in the biological assay. The ureido and thioureido benzoxaboroles (116) exhibited low micromolar inhibitory activities against protozoans, and their derivative, 116a, showed the most activity with an inhibition constant Ki of 0.48 μΜ. Compound 116b containing para-nitrophenyl thiourea exhibited an inhibitory selectivity of 110 times higher towards Leishmania CAs [115]. Compounds 117 and 118, which showed anti-parasitic activity against P. falciparum, T. brucei, T. cruzi or L. donovani, were tested with five different species of Leishmania and found to be new leading compounds for its treatment. The efficacy of these drugs, 117 and 118, was evaluated in vivo against Leishmania major. It was found that 117 suppressed lesion growth upon topical application and 118 reduced the lesion size following an oral administration [116].
Figure 13.
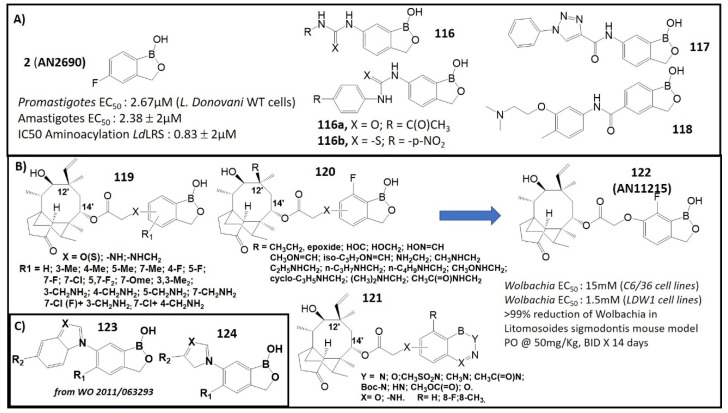
(A) Structures and antileishmanial activity of benzoxaboroles 2 and 116–118(Adapted from [112,115,116]; (B) Structures of pleuromutilin–benzoxaboroles 119–121 and structure and anti-Onchocerca activity of 122 (Adapted from [117,118]); (C) Structures of benzoxaboroles 123 and 124.
4.3. Onchocerciasis (River Blindness) and Lymphatic Filariasis (Elephantiasis)
Onchocerciasis, also known as “river blindness”, is a parasitic disease caused by the filarial worm Onchocerca volvulus and it is transmitted to humans through exposure to repeated bites of infected blackflies of the genus Simulium. Symptoms include severe itching, disfiguring skin conditions, and visual impairment such as permanent blindness. More than 99% of infected people live in African countries [117]. Lymphatic filariasis (commonly known as elephantiasis) is caused by infection with parasite nematodes (roundworms) Wolbachia. bancrofti (which is responsible for 90% of the cases), Brugia. malayi and Brugia. timori. Lymphatic filariasis impairs the lymphatic system and can lead to the abnormal enlargement of body parts, causing pain, severe disability and social stigma. Almost 120 million people in 72 countries worldwide remain threatened by lymphatic filariasis, and they require preventive chemotherapy to stop the spread of this parasitic infection [118].
Pleuromutilin and its derivatives are antibacterial drugs through binding to the peptidyl transfer center (PTC) of the ribosomes and consequently inhibiting protein synthesis of the bacteria [119,120]. Jacobs et al. prepared benzoxaborole analogs of the antibiotic type, known as boronpleuromutilins, by modification of the pleuromutilin core [121]. This modification was focused on linkers of oxygen, nitrogen and sulfur at the 6-position (Figure 13B). A series of benzoxaborole-incorporated pleuromutilins, 119–122, were tested in in vitro assays in the strain of Wolbachia, resulting in encouraging antibacterial potency [121]. Some selected active analogs were analyzed in in vitro absorption, distribution, metabolism, excretion (ADME) and in vivo PK experiments. Compound 7-fluoro-6-oxybenzoxaborole, 122 (AN11251), was identified as a leading compound that showed good in vitro anti-Wolbachia activity and physicochemical and pharmacokinetic properties with high exposure in plasma. This compound was effective in reducing the Wolbachia parasites following oral administration in mice (Figure 13B). The efficacy of 122 in these models suggests more extensive evaluation of this compound, both alone and in combination with other known anti-Wolbachia drugs. Compound 122 may be useful in the treatment of filarial infections or river blindness [121]. In addition, a set of oxaboroles with general structures of 123 and 124 (Figure 13C) were screened against adult worms of B. malayi and obtained moderate results [122].
5. Cryptosporidiosis and Toxoplasmosis
Cryptosporidiosis, also informally called crypto, is a parasitic disease caused by Cryptosporidium parvum (C. parvum) species, a genus of protozoan parasites in the phylum Apicomplexa [123]. Cryptosporidiosis causes high morbidity in developing countries [124]. Toxoplasmosis is a disease caused by infection of the Toxoplasma gondii (T. gondii) parasite [125]. The parasite has two distinct life cycles, where the sexual cycle occurs only in cats, and the definitive host and the asexual cycle occur in other mammals and humans. In the human host, the parasites form tissue cysts, most common in skeletal muscle, myocardium, brain, and eyes; these cysts may remain throughout the life of the host [125]. Despite the seriousness of cryptosporidiosis and toxoplasmosis, interest in the development of new drugs targeting these pathogens has been limited. As described previously, aminoacyl-tRNA synthetases (aaRS) play essential roles in protein synthesis and thus they are the suitable targets for antimicrobial drug design for parasitic diseases [126]. Many benzoxaborole compounds designed by this strategy were screened against Cryptosporidium to discover new potential drugs.
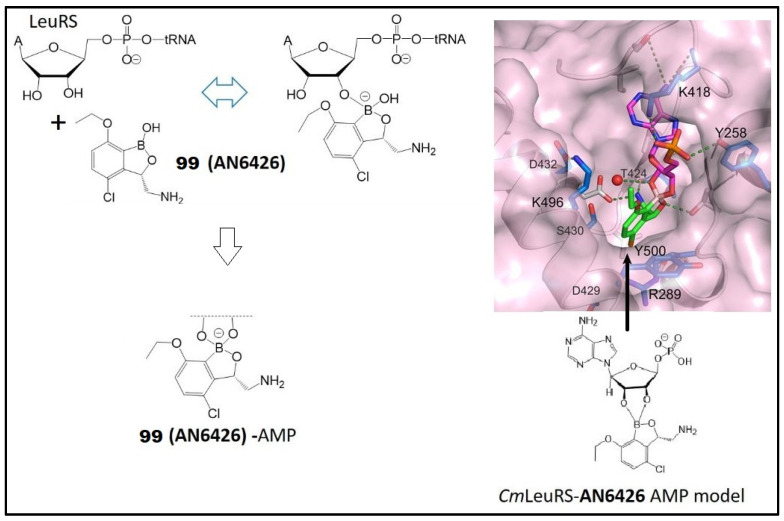
Compounds 3-aminomethyl benzoxaborole (99, AN6426) and its 4-bromo analogue 100 (AN8432) were found to be active against C. parvum, with an IC50 value of 2.2 µΜ for 99 and 6.8 μM for 100, respectively. These activities are comparable to that of nitazoxanide, which is the current standard of care for the treatment of cryptosporidiosis [127]. It was claimed that 99 (AN6426)-AMP adduct can bind to the editing site with a higher affinity than the post-transfer editing substrates (Figure 14). The result was confirmed by in vitro binding experiments and crystal structures of 99 with Cryptosporidium leucyl tRNA synthetase (CmLeuRS) [127]. A stable covalent adduct (spiro product) of 99 (AN6426) in the LeuRS editing was formed, and it may block the aminoacylation reaction. These observations were consistent with those of 99 (AN6426) inhibiting protein synthesis in both Cryptosporidium and Toxoplasma by forming a covalent adduct with tRNALeu [127]. Therefore, benzoxaboroles targeting apicomplexan parasites warrant further development in this area.
Figure 14.
Formation of AN6426-adenosine adduct (left) and crystal structures of post-transfer editing analogues and AN6426 with CmLeuRS (right). (Adapted from [127]).
6. Conclusions
Organoboron compounds have been proven to be attractive candidates as pharmaceutical agents because of their unique physical and chemical properties. Besides being used as boron agents in the treatment of boron neutron capture therapy, organoboron compounds are also essential to treat tropical diseases, including tuberculosis and antifungal activity, malaria, neglected tropical diseases and cryptosporidiosis and toxoplasmosis. The current treatments used for tropical diseases are sub-optimal, and in some cases, there are no drugs available to date. Drug resistance for the clinically used antibiotics and anti-protozoan agents is one of the world’s most serious public health problems. In the last few decades, development in the use of boron derivatives as pharmaceutical agents has produced encouraging strides. The clinical introduction of bortezomib as an anti-cancer agent was followed by benzoxaborole drugs, such as tavaborole and crisaborole, for the treatment of onychomycosis and atopic dermatitis. Anti-infective drugs bearing boron atoms in heterocyclic rings represent a highly interesting field for the pharmaceutical industry, with the potential to obtain drugs with a novel mechanism of action which are effective for the management of infective diseases. It is essential to engage research on anti-tropical diseases with a distinct schedule of short, medium and long-term strategies. This is particularly challenging at the present time, since the COVID-19 crisis has significantly shifted both research attention and governmental resources, and that limited human and financial resources can be used to address such tropical diseases and other diseases [128,129].
Abbreviations
| Mycobacterium tuberculosis | Mtb |
| H. proteasome | Human proteasome |
| MIC | Minimum inhibitory concentration |
| ACT | Artemisinin-based combination therapies |
| aaRSAmino | Acyl tRNA synthetase |
| CQ | Chloroquine |
| ADME | Absorption, distribution, metabolism, and excretion |
| SsrA-tagged protein | Caseinolytic-protease-specific degradation protein |
| RLU | Relative luminescence |
| WT cell lines | Wild-type cell lines |
| aaRS | Aminoacyl-tRNA synthetase |
| ClpP | Caseinolytic proteases |
| TB | Tuberculosis |
| Chloromethyl ketones | CMKs |
| enoyl- reductase | ENR |
| Enoyl-[acyl-carrier-protein] reductase [NADH] | InhA |
| Nicotinamide adenine dinucleotide oxidized | NAD+ |
| Nicotinamide adenine dinucleotide reduced | NADH |
| artemisinin-based combination therapies | ACT |
| Plasmodium Falciparum | P. Falciparum |
| Plasmodium falciparum 3D7 | CQ-sensitive 3D7 |
| structure−activity relationship studies | SARs |
| 3D7 | Chloroquine (CQ) sensitive P. falciparum strain |
| W2 | Chloroquine (CQ) resistant P. falciparum strain |
| D2d | Chloroquine (CQ) resistant P. falciparum strain |
| half-life | t1/2 |
| availability | F |
| WT cells | Wild-type cells |
TOF
Author Contributions
P.S.C. wrote part of the manuscript; Y.Z. (Yinghuai Zhu) created the idea and wrote part of the manuscript; Y.Z. (Yingjun Zhang), H.X. and N.S.H. checked the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by HEC Pharm Group, Dongguan, Guangdong, China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yuan T., Sampson N.S. Hit Generation in TB Drug Discovery: From Genome to Granuloma. Chem. Rev. 2018;118:1887–1916. doi: 10.1021/acs.chemrev.7b00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delves M.J., Migue-Blanco C., Matthews H., Molina I., Yahiya S., Straschil U., Abraham M., Leon M.L., Fischer O.J., Rueda-Zubiaurre A., et al. A high throughtput screen for next-generation leads targeting malaria parasite transmission. Nat. Commun. 2018;9:3805. doi: 10.1038/s41467-018-05777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Why Are Some Tropical Diseases Called “Neglected”? [(accessed on 10 January 2021)]; Available online: http://www.who.int/features/qa/58/en/
- 4.Hotez P.J., Aksoy S., Brindley P.J., Kamhawi S. What constitutes a neglected tropical disease? PLoS Negl. Trop. Dis. 2020;14:e0008001. doi: 10.1371/journal.pntd.0008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanata C.F., Fischer-Walker C.L., Olascoaga A.C., Torres C.X., Aryee M.J., Black R.E. Global causes of diarrheal disease mortality in children <5 years of age: A systematic review. PLoS ONE. 2013;8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das B.C., Thapa P., Karki R., Schinke C., Das S., Kambhampati S., Banerjee S.K., Veldhuizen V.P., Verma A., Weiss L.M., et al. Boron chemicals in diagnosis, therapeutics. Future Med. Chem. 2013;5:653–676. doi: 10.4155/fmc.13.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smedskjaer M.M., Mauro J.C., Youngman R.E., Hogue C.L., Potuzak M., Yue Y. Topological principles of borosilicate glass chemistry. J. Phys. Chem. B. 2011;115:12930–12946. doi: 10.1021/jp208796b. [DOI] [PubMed] [Google Scholar]
- 8.Hosmane N.S., Maguire J.A., Zhu Y., Takagaki M. Boron and Gadolinium Neutron Capture Therapy for Cancer Treatment. World Scientific Publishing Co. Pte. Ltd.; Singapore: 2012. [Google Scholar]
- 9.Wisniak J. Borax, boric acid, boron—From exotic to commodity. Indian J. Chem. Technol. 2005;12:488–500. [Google Scholar]
- 10.Zhu Y., Lin X., Xie H., Li J., Hosmane N.S., Zhang Y. The current status and perspectives of delivery strategy for boronbased drugs. Curr. Med. Chem. 2019;26:5019–5035. doi: 10.2174/0929867325666180904105212. [DOI] [PubMed] [Google Scholar]
- 11.Scholz M., Hey-Hawkins E. Carbaboranes as pharmacophores: Properties, synthesis, application strategies. Chem. Rev. 2011;111:7035–7062. doi: 10.1021/cr200038x. [DOI] [PubMed] [Google Scholar]
- 12.Baker S.J., Tomsho J.W., Benkovic S.J. Boron-containing inhibitors of synthetases. Chem. Soc. Rev. 2011;40:4279–4285. doi: 10.1039/c0cs00131g. [DOI] [PubMed] [Google Scholar]
- 13.Baker S.J., Ding C.Z., Akama T., Zhang Y.-K., Xia Y., Hernandez V. Therapeutic potential of boron containing compounds. Future Med. Chem. 2009;1:1275–1288. doi: 10.4155/fmc.09.71. [DOI] [PubMed] [Google Scholar]
- 14.Adams J., Behnke M., Chen S.W., Cruickshank A.A., Dick L.R., Grenier L., Klunder J.M., Ma Y.T., Plamondon L., Stein R.L. Potent, selective inhibitors of the proteasome: Dipeptidyl boronic acids. Bioorg. Med. Chem. Lett. 1998;8:333–338. doi: 10.1016/S0960-894X(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 15.Adamczyk-Woźniak A., Borys K.M., Sporzyński A. Recent developments in the chemistry and biological applications of benzoxaboroles. Chem. Rev. 2015;115:5224–5247. doi: 10.1021/cr500642d. [DOI] [PubMed] [Google Scholar]
- 16.Anacor Pharmaceuticals FDA Approves Anacor Pharmaceuticals’ KERYDIN® (Tavaborole) Topical Solution, 5% for the Treatment of Onychomycosis of the Toenails. Press Release. Jul 8, 2014.
- 17.Hoy S.M. Crisaborole ointment 2%: A review in mild to moderate atopic dermatitis. Am. J. Clin. Dermatol. 2017;18:837–843. doi: 10.1007/s40257-017-0327-4. [DOI] [PubMed] [Google Scholar]
- 18.Scifinder, Version 2019. Chemical Abstracts Service; Columbus, OH, USA: 2019. [(accessed on 15 March 2019)]. [Google Scholar]
- 19.Zhang P., Ma S. Recent development of leucyl-tRNA synthetase inhibitors as antimicrobial agents. Med. Chem. Commun. 2019;10:1329–1341. doi: 10.1039/C9MD00139E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang F., Zhu M., Zhang J., Zhou H. Synthesis of biologically active boron-containing compounds. Med. Chem. Comm. 2018;9:201. doi: 10.1039/C7MD00552K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dembitsky V.M., Quntar A.A.A., Srebnik M. Natural and Synthetic Small Boron-Containing Molecules as Potential Inhibitors of Bacterial and Fungal Quorum Sensing. Chem. Rev. 2011;111:209–237. doi: 10.1021/cr100093b. [DOI] [PubMed] [Google Scholar]
- 22.Silva M.P., Saraiva L., Pinto M., Sousa M.E. Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications. Molecules. 2020;25:4323. doi: 10.3390/molecules25184323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. [(accessed on 11 February 2021)]; Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf.
- 24.Ernst J.D. The immunological life cycle of tuberculosis. Nat. Rev. Immunol. 2012;12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 25.Vjecha M.J., Tiberi S., Zumla A. Accelerating the development of therapeutic strategies for drug-resistant tuberculosis. Nat. Rev. Drug. Discov. 2018;17:607–608. doi: 10.1038/nrd.2018.28. [DOI] [PubMed] [Google Scholar]
- 26.Chetty S., Ramesh M., Singh-Pillay A., Soliman M.E. Recent advancements in the development of anti-tuberculosis drugs. Bioorg. Med. Chem. Lett. 2017;27:370–386. doi: 10.1016/j.bmcl.2016.11.084. [DOI] [PubMed] [Google Scholar]
- 27.Torssell K. Bromination of tolylboronic acids according to Wohl-Ziegler. Ark. Kemi. 1957;10:507–511. [Google Scholar]
- 28.Adamczyk-Wozniak A., Cyranski M.K., Zubrowska A., Sporzynski A. Benzoxaboroles—Old compounds with new applications. J. Organomet. Chem. 2009;694:3533–3541. doi: 10.1016/j.jorganchem.2009.07.022. [DOI] [Google Scholar]
- 29.Mereddy G.R., Chakradhar A., Rutkoski R.M., Jonnalagadda S.C. Benzoboroxoles: Synthesis and applications in medicinal chemistry. J. Organomet. Chem. 2018;865:12–22. doi: 10.1016/j.jorganchem.2018.03.017. [DOI] [Google Scholar]
- 30.Nocentini A., Supuran C.T., Winum J.Y. Benzoxaborole compounds for therapeutic uses: A patent review (2010–2018) Expert. Opin. Ther. Pat. 2018;28:493–504. doi: 10.1080/13543776.2018.1473379. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Zhu M., Lin Y., Zhou H. The synthesis of benzoxaboroles and their applications in medicinal chemistry. Sci. China Chem. 2013;56:1372–1381. doi: 10.1007/s11426-013-4981-y. [DOI] [Google Scholar]
- 32.Rock F.L., Mao W., Yaremchuk A., Tukalo M., Crepin T., Zhou H., Zhang Y.K., Hernandez V., Akama T., Baker S.J., et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759–1761. doi: 10.1126/science.1142189. [DOI] [PubMed] [Google Scholar]
- 33.Gupta A.K., Simpson F.C. New therapeutic options for onychomycosis. Expert. Opin. Pharmacother. 2012;13:1131–1142. doi: 10.1517/14656566.2012.681779. [DOI] [PubMed] [Google Scholar]
- 34.Sharma N., Sharma D. An upcoming drug for onychomycosis: Tavaborole. J. Pharmacol. Pharmacother. 2015;6:236–239. doi: 10.4103/0976-500X.171870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee E.Y., Kim S., Kim M.H. Aminoacyl-tRNA synthetases, therapeutic targets for infectious diseases. Biochem. Pharmacol. 2018;154:424–434. doi: 10.1016/j.bcp.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seiradake E., Mao W., Hernandez V., Baker S.J., Plattner J.J., Alley M.R., Cusack S. Crystal structures of the human and fungal cytosolic Leucyl-tRNA synthetase editing domains: A structural basis for the rational design of antifungal benzoxaboroles. J. Mol. Biol. 2009;390:196–207. doi: 10.1016/j.jmb.2009.04.073. [DOI] [PubMed] [Google Scholar]
- 37.Alam M.A., Arora K., Gurrapu S., Jonnalagadda S.K., Nelson G.L., Kiprof P., Jonnalagadda S.C., Mereddy V.R. Synthesis and evaluation of functionalized benzoboroxoles as potential anti-tuberculosis agents. Tetrahedron. 2016;72:3795–3801. doi: 10.1016/j.tet.2016.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palencia A., Li X., Bu W., Choi W., Ding C.Z., Easom E.E., Feng L., Hernandez V., Houston P., Liu L., et al. Discovery of Novel Oral Protein Synthesis Inhibitors of Mycobacterium Tuberculosis That Target Leucyl-tRNA Synthetase. Antimicrob. Agents Chemother. 2016;60:6271–6280. doi: 10.1128/AAC.01339-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y., Liao J., Zhu B., Wang E.D., Ding J. Crystal structures of the editing domain of Escherichia coli leucyl-tRNA synthetase and its complexes with Met and Ile reveal a lock-and-key mechanism for amino acid discrimination. Biochem. J. 2006;394:399–407. doi: 10.1042/BJ20051249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X., Hernandez V., Rock F.L., Choi W., Mak Y.S.L., Mohan M., Mao W., Zhou Y., Easom E.E., Plattner J.J., et al. Discovery of a Potent and Specific M. tuberculosis Leucyl-tRNA Synthetase Inhibitor: (S)-3-(Aminomethyl)-4-chloro-7-(2-hydroxyethoxy)benzo[c][1,2]oxaborol-1(3H)-ol (GSK656) J. Med. Chem. 2017;60:8011–8026. doi: 10.1021/acs.jmedchem.7b00631. [DOI] [PubMed] [Google Scholar]
- 41.Vshyvenko S., Clapson M.L., Suzuki I., Hall D.G. Characterization of the dynamic equilibrium between closed and open forms of the benzoxaborole pharmacophore. ACS Med. Chem. Lett. 2016;7:1097–1101. doi: 10.1021/acsmedchemlett.6b00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. [(accessed on 10 March 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03075410.
- 43.Patel N., O’Malley T., Zhang Y.K., Xia Y., Sunde B., Flint L., Korkegian A., Loerger T.R., Sacchettini J., Alley M.R.K., et al. A Novel 6-Benzyl Ether Benzoxaborole Is Active against Mycobacterium tuberculosis in vitro. Antimicrob. Agents Chemother. 2017;61:pii: e01205-17. doi: 10.1128/AAC.01205-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korkegian A., O’Malley T., Xia Y., Zhou Y., Carter D.S., Sunde B., Flint L., Thompson D., Ioerger T.R., Sacchettini J., et al. The 7-phenyl benzoxaborole series is active against Mycobacterium tuberculosis. Tuberculosis. 2018;108:96–98. doi: 10.1016/j.tube.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yano T., Rahimian M., Aneja K.K., Schechter N.M., Rubin H., Scott C.P. Mycobacterium tuberculosis type II NADH-menaquinone oxidoreductase catalyzes electron transfer through a two-site ping-pong mechanism and has two quinone-binding sites. Biochemistry. 2014;53:1179–1190. doi: 10.1021/bi4013897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee A.S., Teo A.S., Wong S.Y. Novel mutations in ndh in isoniazid-resistant Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 2001;45:2157–2159. doi: 10.1128/AAC.45.7.2157-2159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sena F.V., Batista A.P., Catarino T., Brito J.A., Archer M., Viertler M., Madl T., Cabrita E.J., Pereira M.M. Type-II NADH:quinone oxidoreductase from Staphylococcus aureus has two distinct binding sites and is rate limited by quinone reduction. Mol. Microbiol. 2015;98:272–288. doi: 10.1111/mmi.13120. [DOI] [PubMed] [Google Scholar]
- 48.Guy C.S., Murray K., Gibson M.I., Fullam E. Dimeric benzoboroxoles for targeted activity against Mycobacterium tuberculosis. Org. Biomol. Chem. 2019;17:9524–9528. doi: 10.1039/C9OB02222H. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Y., Wu G., Zhu X., Ma Y., Zhao X., Li Y., Yuan Y., Yang J., Yu S., Shao F., et al. Synthesis, in vitro and in vivo Biological Evaluation, and Comprehensive Understanding of Structure—Activity Relationships of Dipeptidyl Boronic Acid Proteasome Inhibitors Constructed from β-Amino Acids. J. Med. Chem. 2010;53:8619–8626. doi: 10.1021/jm1009742. [DOI] [PubMed] [Google Scholar]
- 50.Han L., Wen Y., Li R., Xu B., Ge Z., Wang X., Cheng T., Cui J., Li R. Synthesis and biological activity of peptide proline-boronic acids as proteasome inhibitors. Bioorg. Med. Chem. 2017;25:4031–4044. doi: 10.1016/j.bmc.2017.05.049. [DOI] [PubMed] [Google Scholar]
- 51.Chen D., Frezza M., Schmitt S., Kanwar J., Dou Q.P. Bortezomib as the first proteasome inhibitor anticancer drug: Current status and future perspectives. Curr. Cancer Drug Targets. 2011;11:239–253. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groll M., Berkers C.R., Ploegh H.L., Ovaa H. Crystal structure of the boronic acid-based proteasome inhibitor bortezomib in complex with the yeast 20S proteasome. Structure. 2006;14:451–456. doi: 10.1016/j.str.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 53.Bonvini P., Zorzi E., Basso G., Rosolen A. Bortezomib-mediated 26S proteasome inhibition causes cell-cycle arrest and induces apoptosis in CD-30 anaplastic large cell lymphoma. Leukemia. 2007;21:838–842. doi: 10.1038/sj.leu.2404528. [DOI] [PubMed] [Google Scholar]
- 54.Chauhan D., Tian Z., Zhou B., Kuhn D., Orlowski R., Raje N., Richardson P., Anderson K.C. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. Clin. Cancer. Res. 2011;17:5311–5321. doi: 10.1158/1078-0432.CCR-11-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brötz-Oesterhelt H., Sass P. Bacterial caseinolytic proteases as novel targets for antibacterial treatment. Int. J. Med. Microbiol. 2014;304:23–30. doi: 10.1016/j.ijmm.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Raju R.M., Jedrychowski M.P., Wei J., Pinkham J.T., Park A.S., O’Brien K., Rehren G., Schnappinger D., Gygi S.P., Rubin E.J. Post-translational regulation via Clp protease is critical for survival of Mycobacterium tuberculosis. PLoS Pathog. 2014;10:e1003994. doi: 10.1371/journal.ppat.1003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keiler K.C. Biology of trans-translation. Annu. Rev. Microbiol. 2008;62:133–151. doi: 10.1146/annurev.micro.62.081307.162948. [DOI] [PubMed] [Google Scholar]
- 58.Raju R.M., Unnikrishnan M., Rubin D.H., Krishnamoorthy V., Kandror O., Akopian T.N., Goldberg A.L., Rubin E.J. Mycobacterium tuberculosis ClpP1 and ClpP2 function together in protein degradation and are required for viability in vitro and during infection. PLoS Pathog. 2012;8:e1002511. doi: 10.1371/journal.ppat.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moreira W., Ngan G.J., Low J.L., Poulsen A., Chia B.C., Ang M.J., Yap A., Fulwood J., Lakshmanan U., Lim J., et al. Target mechanism-based whole-cell screening identifies bortezomib as an inhibitor of caseinolytic protease in mycobacteria. mBio. 2015;6:e00253-15. doi: 10.1128/mBio.00253-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bogyo M., Wang E.W. Proteasome inhibitors: Complex tools for a complex enzyme. Curr. Top. Microbiol. Immunol. 2002;268:185–208. doi: 10.1007/978-3-642-59414-4_8. [DOI] [PubMed] [Google Scholar]
- 61.Moreira W., Santhanakrishnan S., Dymock B.W., Dick T. Bortezomib Warhead-Switch Confers Dual Activity against Mycobacterial Caseinolytic Protease and Proteasome and Selectivity against Human Proteasome. Front. Microbiol. 2017;27:746. doi: 10.3389/fmicb.2017.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreira W., Santhanakrishnan S., Ngan G.J.Y., Low C.B., Sangthongpitag K., Poulsen A., Dymock B.W., Dick T. Towards Selective Mycobacterial ClpP1P2 Inhibitors with Reduced Activity against the Human Proteasome. Antimicrob. Agents Chemother. 2017;61:e02307-16. doi: 10.1128/AAC.02307-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 1997;23:3–26. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- 64.Fernandes G.F.S., Denny W.A., Dos Santos J.L. Boron in drug design: Recent advances in the development of new therapeutic agents. Eur. J. Med. Chem. 2019;179:791–804. doi: 10.1016/j.ejmech.2019.06.092. [DOI] [PubMed] [Google Scholar]
- 65.Grassberger M.A., Turnowsky F., Hildebrandt J. Preparation and antibacterial activities of new 1,2,3-diazaborine derivatives and analogs. J. Med. Chem. 1984;27:947–953. doi: 10.1021/jm00374a003. [DOI] [PubMed] [Google Scholar]
- 66.Baldock C., Rafferty J.B., Sedelnikova S.E., Baker P.J., Stuitje A.R., Slabas A.R., Hawkes T.R., Rice D.W. Mechanism of Drug Action Revealed by Structural Studies of Enoyl Reductase. Science. 1996;274:2107–2110. doi: 10.1126/science.274.5295.2107. [DOI] [PubMed] [Google Scholar]
- 67.De Boer G.J., Pielage G.J., Nijkamp H.J., Slabas A.R., Rafferty J.B., Baldock C., Rice D.W., Stuitje A.R. Molecular genetic analysis of enoyl-acyl carrier protein reductase inhibition by diazaborine. Mol. Microbiol. 1999;31:443–450. doi: 10.1046/j.1365-2958.1999.01182.x. [DOI] [PubMed] [Google Scholar]
- 68.Xia Y., Zhou Y., Carter D.S., McNeil M.B., Choi W., Halladay J., Berry P.W., Mao W., Hernandez V., O’Malley T., et al. Discovery of a cofactor-independent inhibitor of Mycobacterium tuberculosis InhA. Life Sci. Alliance. 2018;1:e201800025. doi: 10.26508/lsa.201800025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takayama K., Wang C., Besra G.S. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 2005;18:81–101. doi: 10.1128/CMR.18.1.81-101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davis M.C., Franzblau S.G., Martin A.R. Syntheses and evaluation of benzodiazaborine compounds against M. tuberculosis H37Rv in vitro. Bioorg. Med. Chem. Lett. 1998;8:843–846. doi: 10.1016/S0960-894X(98)00126-7. [DOI] [PubMed] [Google Scholar]
- 71.Kanichar D., Roppiyakuda L., Kosmowska E., Faust M.A., Tran K.P., Chow F., Buglo E., Groziak M.P., Sarina E.A., Olmstead M.M. Synthesis, Characterization, and Antibacterial Activity of Structurally Complex 2-Acylated 2,3,1-Benzodiazaborines and Related Compounds. Chem. Biodivers. 2014;11:1381–1397. doi: 10.1002/cbdv.201400007. [DOI] [PubMed] [Google Scholar]
- 72.Hicks J.W., Kyle C.B., Vogels C.M., Wheaton S.L., Baerlocher F.J., Decken A., Westcott S.A. Synthesis, characterization, and antifungal activity of boron-containing thiosemicarbazones. Chem. Biodivers. 2008;5:2415–2422. doi: 10.1002/cbdv.200890206. [DOI] [PubMed] [Google Scholar]
- 73.Rajan R., Budihal V., Renold P., Stierli D. Preparation of Boron Containing Fungicides and Their Use in Compns. and Methods for the Control and/or Prevention of Microbial Infection in Plants. WO2014167133. MedChemComm. 2018;9:201–211.
- 74.Moreira W., Aziz D.B., Dick T. Boromycin Kills Mycobacterial Persisters without Detectable Resistance. Front. Microbiol. 2016;7:199. doi: 10.3389/fmicb.2016.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. [(accessed on 27 April 2021)]; Available online: https://www.who.int/publications/i/item/9789240015791.
- 76.Ouji M., Augereau J.M., Paloque L., Benoit-Vical F. Plasmodium falciparum resistance to artemisinin-based combination therapies: A sword of Damocles in the path toward malaria elimination. Parasite. 2018;25:24. doi: 10.1051/parasite/2018021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. [(accessed on 10 February 2021)]; Available online: https://www.cdc.gov/malaria/about/biology/index.html.
- 78.Artesunate. [(accessed on 10 February 2021)]; Available online: http://apps.who.int/medicinedocs/en/d/Jh2922e/2.5.11.html.
- 79.Sibley C.H. Understanding artemisinin resistance. Science. 2015;347:373–374. doi: 10.1126/science.aaa4102. [DOI] [PubMed] [Google Scholar]
- 80.Heller L.E., Roepe P.D. Artemisinin-Based Antimalarial Drug Therapy: Molecular Pharmacology and Evolving Resistance. Trop. Med. Infect. Dis. 2019;4:89. doi: 10.3390/tropicalmed4020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacobs R.T., Plattner J.J., Keenan M. Boron-based drugs as antiprotozoals. Curr. Opin. Infect. Dis. 2011;24:586–592. doi: 10.1097/QCO.0b013e32834c630e. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y.-K., Plattner J.J., Freund Y.R., Easom E.E., Zhou Y., Gut J., Rosenthal P.J., Waterson D., Gamo F., Angulo-Barturen I., et al. Synthesis and structure-activity relationships of novel benzoxaboroles as a new class of antimalarial agents. Bioorg. Med. Chem. Lett. 2011;21:644–651. doi: 10.1016/j.bmcl.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y.-K., Plattner J.J., Easom E.E., Waterson D., Ge M., Li Z., Li L., Jian Y. An efficient synthesis for a new class antimalarial agent, 7-(2-carboxyethyl)-1,3-dihydro-1-hydroxy-2,1-benzoxaborole. Tetrahedron Lett. 2011;52:3909–3911. doi: 10.1016/j.tetlet.2011.05.088. [DOI] [Google Scholar]
- 84.Zhang Y.-K., Plattner J.J., Freund Y.R., Easom E.E., Zhou Y., Huchen L.Y., Zhou H., Waterson D., Gamo F., Sanz L.M., et al. Benzoxaborole antimalarial agents. Part 2: Discovery of fluoro-substituted 7-(2-carboxyethyl)-1,3-dihydro-1-hydroxy-2,1-benzoxaboroles. Bioorg. Med. Chem. Lett. 2012;22:1299–1307. doi: 10.1016/j.bmcl.2011.12.096. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y.-K., Plattner J.J., Easom E.E., Jacobs R.T., Guo D., Sanders V., Freund Y.R., Campo B., Rosenthal P.J., Bu W., et al. Benzoxaborole antimalarial agents. Part 4. Discovery of potent 6-(2-(alkoxycarbonyl)pyrazinyl-5-oxy)-1,3-dihydro-1-hydroxy-2,1-benzoxaboroles. J. Med. Chem. 2015;58:5344–5354. doi: 10.1021/acs.jmedchem.5b00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y.-K., Plattner J.J., Easom E.E., Jacobs R.T., Guo D., Freund Y.R., Berry P., Ciaravino V., Erve J.C.L., Rosenthal P.J., et al. Benzoxaborole Antimalarial Agents. Part 5. Lead Optimization of Novel Amide Pyrazinyloxy Benzoxaboroles and Identification of a Preclinical Candidate. J. Med. Chem. 2017;60:5889–5908. doi: 10.1021/acs.jmedchem.7b00621. [DOI] [PubMed] [Google Scholar]
- 87.Hu Q.H., Liu R.J., Fang Z.P., Zhang J., Ding Y.Y., Tan M., Wang M., Pan W., Zhou H.C., Wang E.D. Discovery of a potent benzoxaborole-based anti-pneumococcal agent targeting leucyl-tRNA synthetase. Sci. Rep. 2013;3:2475. doi: 10.1038/srep02475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sonoiki E., Palencia A., Guo D., Ahyong V., Dong C., Li X., Hernandez V.S., Zhang Y.K., Choi W., Gut J., et al. Antimalarial Benzoxaboroles Target Plasmodium falciparum Leucyl-tRNA Synthetase. Antimicrob. Agents Chemother. 2016;60:4886–4895. doi: 10.1128/AAC.00820-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hernandez V.S., Ding C., Plattner J.J., Alley M.R.K., Rock F., Zhang S., Easom E., Li X., Zhou D. Benzoxaborole Derivatives for Treating Bacterial Infections. WO2012033858A2. 2012 Mar 15;
- 90.Sonoiki E., Ng C.L., Lee M.C., Guo D., Zhang Y.K., Zhou Y., Alley M.R.K., Ahyong V., Sanz L.M., Lafuente-Monasterio M.J., et al. A potent antimalarial benzoxaborole targets a Plasmodium falciparum cleavage and polyadenylation specificity factor homologue. Nat. Commun. 2017;8:14574. doi: 10.1038/ncomms14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mandel C.R., Kaneko S., Zhang H., Gebauer D., Vethantham V., Manley J.L., Tong L. Polyadenylation factor CPSF-73 is the pre-mRNA 3′-end-processing endonuclease. Nature. 2006;444:953–956. doi: 10.1038/nature05363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.The WHO List of 17 Neglected Tropical Diseases. [(accessed on 28 April 2021)]; Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/overview/progress-dashboard-2011-2020.
- 93.Peacock L., Cook S., Ferris V., Bailey M., Gibson W. The life cycle of Trypanosoma (Nannomonas) congolense in the tsetse fly. Parasites Vectors. 2012;5:109. doi: 10.1186/1756-3305-5-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ding D., Zhao Y., Meng Q., Xie D., Nare B., Chen D., Bacchi C.J., Yarlett N., Zhang Y.-K., Hernandez V., et al. Discovery of Novel Benzoxaborole-Based Potent Antitrypanosomal Agents. ACS Med. Chem. Lett. 2010;1:165–169. doi: 10.1021/ml100013s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nare B., Wring S., Bacchi C., Beaudet B., Bowling T., Brun R., Chen D., Ding C., Freund Y., Gaukel E., et al. Discovery of Novel Orally Bioavailable Oxaborole 6-Carboxamides That Demonstrate Cure in a Murine Model of Late-Stage Central Nervous System African Trypanosomiasis. Antimicrobic. Agents Chemother. 2010;54:4379–4388. doi: 10.1128/AAC.00498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doan K.M., Wring S.A., Shampine L.J., Jordan K.H., Bishop J.P., Kratz J., Yang E., Serabjit-Singh C.J., Adkison K.K., Polli J.W. Steady-state brain concentrations of antihistamines in rats: Interplay of membrane permeability, P-glycoprotein efflux and plasma protein binding. Pharmacology. 2004;72:92–98. doi: 10.1159/000079137. [DOI] [PubMed] [Google Scholar]
- 97.Jacobs R.T., Plattner J.J., Nare B., Wring S.A., Chen D., Freund Y., Gaukel E.G., Orr M.D., Perales J.B., Jenks M., et al. Benzoxaboroles: A new class of potential drugs for human African trypanosomiasis. Future Med. Chem. 2011;3:1259–1278. doi: 10.4155/fmc.11.80. [DOI] [PubMed] [Google Scholar]
- 98.Jacobs R.T., Nare B., Wring S.A., Orr M.D., Chen D., Sligar J.M., Jenks M.X., Noe R.A., Bowling T.S., Mercer L.T., et al. SCYX-7158, an orally-active benzoxaborole for the treatment of stage 2 human African trypanosomiasis. PLoS Negl. Trop. Dis. 2011;5:e1151. doi: 10.1371/journal.pntd.0001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Drugs for Neglected Diseases Initiative (Dndi). Dndi Announces Successful Completion of SCYX-7158 Phase I Study for Treatment of Sleeping Sicknes. [(accessed on 11 March 2021)];2015 Available online: https://www.news-medical.net/news/20150909/DNDi-announces-successful-completion-of-SCYX-7158-Phase-I-study-for-treatment-of-sleeping-sickness.aspx.
- 100. [(accessed on 20 April 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03087955.
- 101.Aponte J.C., Verastegui M., Malaga E., Zimic M., Quiliano M., Vaisberg A.J., Gilman R.H., Hammond G.B. Synthesis, cytotoxicity, and anti-Trypanosoma cruzi activity of new chalcones. J. Med. Chem. 2008;51:6230–6234. doi: 10.1021/jm800812k. [DOI] [PubMed] [Google Scholar]
- 102.Qiao Z., Wang Q., Zhang F., Wang Z., Bowling T., Nare B., Jacobs R.T., Zhang J., Ding D., Liu Y., et al. Chalcone–Benzoxaborole Hybrid Molecules as Potent Antitrypanosomal Agents. J. Med. Chem. 2012;55:3553–3557. doi: 10.1021/jm2012408. [DOI] [PubMed] [Google Scholar]
- 103.Wu P., Zhang J., Meng Q., Nare B., Jacobs R.T., Zhou H. Novel pyrrolobenzoxaboroles: Design, synthesis, and biological evaluation against Trypanosoma brucei. Eur. J. Med. Chem. 2014;81:59–75. doi: 10.1016/j.ejmech.2014.04.079. [DOI] [PubMed] [Google Scholar]
- 104.Gumbo M., Beteck R.M., Mandizvo T., Seldon R., Warner D.F., Hoppe H.C., Isaacs M., Laming D., Tam C.C., Cheng L.W., et al. Cinnamoyl-Oxaborole Amides: Synthesis and Their in Vitro Biological Activity. Molecules. 2018;23:2038. doi: 10.3390/molecules23082038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ding D., Meng Q., Gao G., Zhao Y., Wang Q., Nare B., Jacobs R.T., Rock F., Alley M.R., Plattner J.J., et al. Design, synthesis, and structure-activity relationship of Trypanosoma brucei leucyl-tRNA synthetase inhibitors as antitrypanosomal agents. J. Med. Chem. 2011;54:1276–1287. doi: 10.1021/jm101225g. [DOI] [PubMed] [Google Scholar]
- 106. [(accessed on 29 April 2021)]; Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
- 107.Torres-Guerrero E., Quintanilla-Cedillo M.R., Ruiz-Esmenjaud J., Arenas R. Leishmaniasis: A review. F1000Res. 2017;6:750. doi: 10.12688/f1000research.11120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sundar S., Pandey K., Thakur C.P., Jha T.K., Das V.N.R., Verma N., Lal C.S., Verma D., Alam S., Das P. Efficacy and safety of amphotericin B emulsion versus liposomal formulation in Indian patients with visceral leishmaniasis: A randomized, open-label study. PLoS Negl. Trop. Dis. 2014;8:e3169. doi: 10.1371/journal.pntd.0003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tiwari N., Gedda M.R., Tiwari V.K., Singh S.P., Singh R.K. Limitations of current therapeutic options, possible drug targets and scope of natural products in control of leishmaniasis. Mini. Rev. Med. Chem. 2018;18:26–41. doi: 10.2174/1389557517666170425105129. [DOI] [PubMed] [Google Scholar]
- 110.Pandey B.D., Pandey K., Kaneko O., Yanagi T., Hirayama K. Relapse of visceral leishmaniasis after miltefosine treatment in a Nepalese patient. Am. J. Trop. Med. Hyg. 2009;80:580–582. doi: 10.4269/ajtmh.2009.80.580. [DOI] [PubMed] [Google Scholar]
- 111.WHO. Accelerating work to overcome the global impact of neglected tropical diseases: A roadmap for implementation. WHO/HTM/NTD/2012. World Health Organization, Geneva, Switzerland. [(accessed on 22 February 2021)];2012 Available online: http://www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf.
- 112.Manhas R., Tandon S., Sen S.S., Tiwari N., Munde M., Madhubala R. Leishmania donovani Parasites Are Inhibited by the Benzoxaborole AN2690 Targeting Leucyl-tRNA Synthetase. Antimicrob. Agents Chemother. 2018;62:e00079-18. doi: 10.1128/AAC.00079-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vermelho A.B., Capaci G.R., Rodrigues I.A., Cardoso V.S., MariaMazotto A., Supuran C.T. Carbonic anhydrases from Trypanosoma and Leishmania as anti-protozoan drug targets. Bioorg. Med. Chem. 2017;25:1543–1555. doi: 10.1016/j.bmc.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 114.Capasso C., Supuran C.T. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin. Ther. Targets. 2015;19:1689–1704. doi: 10.1517/14728222.2015.1067685. [DOI] [PubMed] [Google Scholar]
- 115.Nocentini A., Cadoni R., Dumy P., Supuran C.T., Winum J.-Y. Carbonic anhydrases from Trypanosoma cruzi and Leishmania donovani chagasi are inhibited by benzoxaboroles. Enzyme Inhib. Med. Chem. 2018;33:286–289. doi: 10.1080/14756366.2017.1414808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van Bocxlaer K., Gaukel E., Hauser D., Park S.H., Schock S., Yardley V., Randolph R., Plattner J.J., Merchant T., Croft S.L., et al. Topical Treatment for Cutaneous Leishmaniasis: Dermato-Pharmacokinetic Lead Optimization of Benzoxaboroles. Antimicrob. Agents Chemother. 2018;62:e02419-17. doi: 10.1128/AAC.02419-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. [(accessed on 21 April 2021)]; Available online: https://www.who.int/blindness/partnerships/onchocerciasis_disease_information/en/
- 118. [(accessed on 21 April 2021)]; Available online: https://www.who.int/lymphatic_filariasis/en/
- 119.Kavanagh F., Hervey A., Robbins W.J. Antibiotic substances from basidiomycetes: IX. Drosophila subtarata. (batsch ex fr.) quel. Proc. Natl. Acad. Sci. USA. 1952;38:555–560. doi: 10.1073/pnas.38.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schlunzen F., Pyetan E., Fucini P., Yonath A., Harms J.M. Inhibition of peptide bond formation by pleuromutilins: The structure of the 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin. Mol. Microbiol. 2004;54:1287–1294. doi: 10.1111/j.1365-2958.2004.04346.x. [DOI] [PubMed] [Google Scholar]
- 121.Jacobs R.T., Lunde C.S., Freund Y.R., Hernandez V., Li X., Xia Y., Carter D.S., Berry P.W., Halladay J., Rock F., et al. Boron-Pleuromutilins as Anti-Wolbachia Agents with Potential for Treatment of Onchocerciasis and Lymphatic Filariasis. J. Med. Chem. 2019;62:2521–2540. doi: 10.1021/acs.jmedchem.8b01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y.-K., Zhou H., Ding C., Plattner J.J., Freund Y. Boron-Containing Small Molecules as Antihelminth Agents. WO201106. 2011 May 26;
- 123. [(accessed on 29 April 2021)]; Available online: https://en.wikipedia.org/wiki/Cryptosporidiosis.
- 124.WHO. World Health Organization. [(accessed on 1 March 2021)];2018 Available online: https://www.who.int/water_sanitation_health/gdwqrevision/cryptodraft2.pdf.
- 125.Dubey J.P. In: Chapter 1—The History and Life Cycle of Toxoplasma Gondii in Toxoplasma Gondii. 2nd ed. Weiss L.M., editor. Academic Press; Cambridge, MA, USA: 2014. pp. 1–17. [Google Scholar]
- 126.Lv P.-C., Zhu H.-L. Aminoacyl-tRNA synthetase inhibitors as potent antibacterials. Curr. Med. Chem. 2012;19:3550–3563. doi: 10.2174/092986712801323199. [DOI] [PubMed] [Google Scholar]
- 127.Palencia A., Liu R.J., Lukarska M., Gut J., Bougdour A., Touquet B., Wang E.D., Li X., Alley M.R., Freund Y.R., et al. Cryptosporidium and Toxoplasma Parasites Are Inhibited by a Benzoxaborole Targeting Leucyl-tRNA Synthetase. Antimicrob. Agents Chemother. 2016;60:5817–5827. doi: 10.1128/AAC.00873-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prudêncio M., Costa J.C. Research funding after COVID-19. Nat. Microbiol. 2020;5:986. doi: 10.1038/s41564-020-0768-z. [DOI] [PubMed] [Google Scholar]
- 129.Burki T.K. Cuts in cancer research funding due to COVID-19. Lancet Oncol. 2021;22:E6. doi: 10.1016/S1470-2045(20)30749-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.