Figure 1.
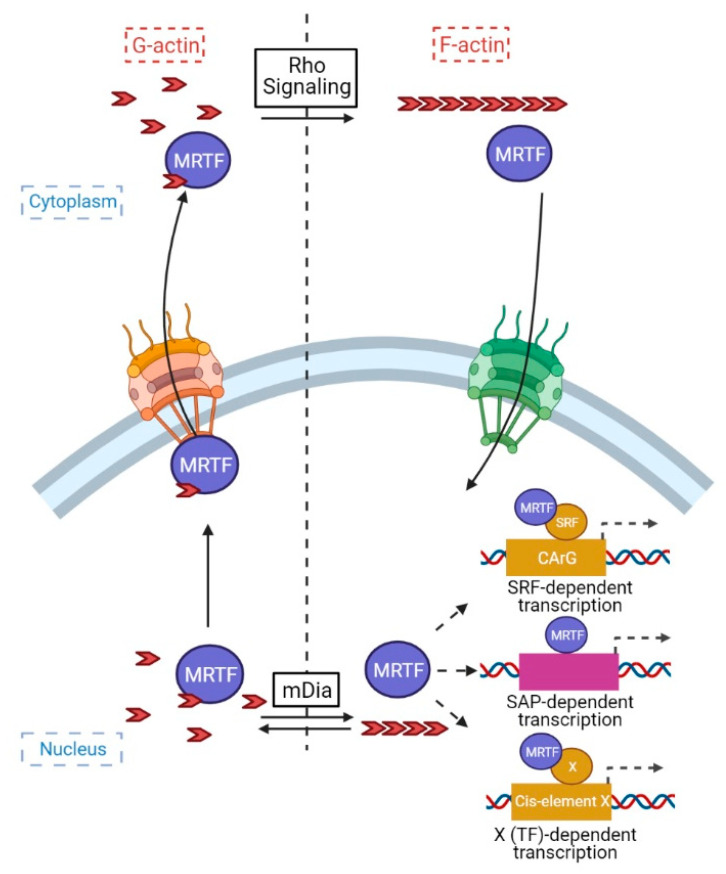
The regulation of MRTF subcellular localization and transcriptional activity by the actin cytoskeleton MRTF-A/B are transcriptional coactivators regulated by F/G actin ratio. Cytosolic G-actin binds MRTF masking a Nuclear Localization Sequence (NLS) (see Figure 2). Activation of small Rho GTPases promotes F-actin polymerization, thereby reducing the cytosolic G-actin pool, and causing G-actin to dissociate from MRTF to unmask the NLS, which enables importin α/β-dependent nuclear translocation. In the nucleus (1) MRTF binds SRF forming the MRTF/SRF complex which induces the transcription of various genes (SRF-dependent transcription); (2) MRTF can independently facilitate transcription of genes via its SAP domain (SAP-dependent transcription); and (3) MRTF can bind other TFs (e.g., smad3, TAZ) which induce transcription via alternative non-CArG dependent cis-elements. Additionally, the nuclear F/G actin ratio regulates MRTF function. Increased mDia activity enhances MRTF/SRF transcriptional activity, while nuclear G-actin binding to MRTF supports CMR1-dependent nuclear export. Together, the actin cytoskeleton acts as an integral regulator of MRTF-dependent gene expression (dotted arrows). (Created with BioRender.com, accessed on 15 April 2021).