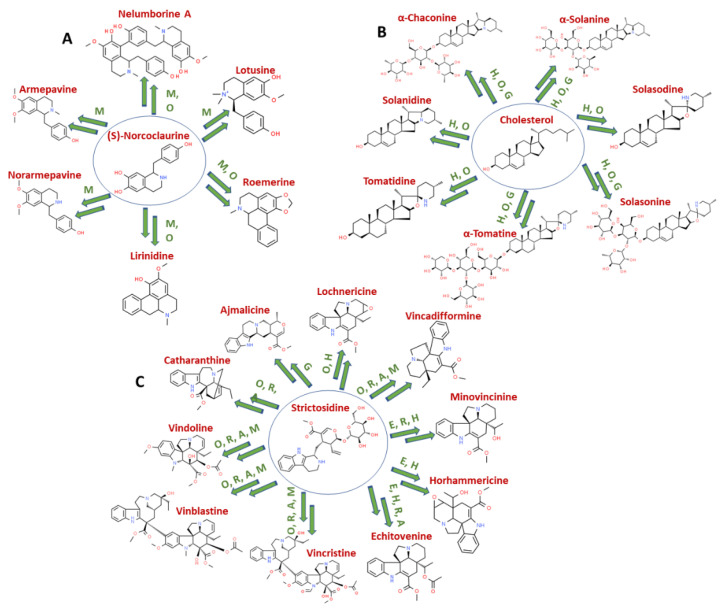
Figure 1.
Examples of alkaloids biosynthesized from the common skeleton. Multiple alkaloids are biosynthesized from the common skeleton represented inside the blue circle in different plant species. (A) Benzylisoquinoline alkaloids in Sacred lotus; (B) Steroidal alkaloids and their glycosides in tomato, potato, and eggplant; (C) Terpene indole alkaloids in C. roseus. The common skeleton undergoes multiple enzymatic conversions (M = methylation, O = oxidation, R = reduction, G = glycosylation, A = acetylation, H = hydroxylation, and E = epoxidation) represented by multiple arrows to form a variety of alkaloids. Key enzymatic reactions that are reported have been mentioned beside the arrows. The chemical structures of alkaloids are drawn from “ChemSpider: the Free Chemical Database”.