Figure 1. Selection of the Study Population.
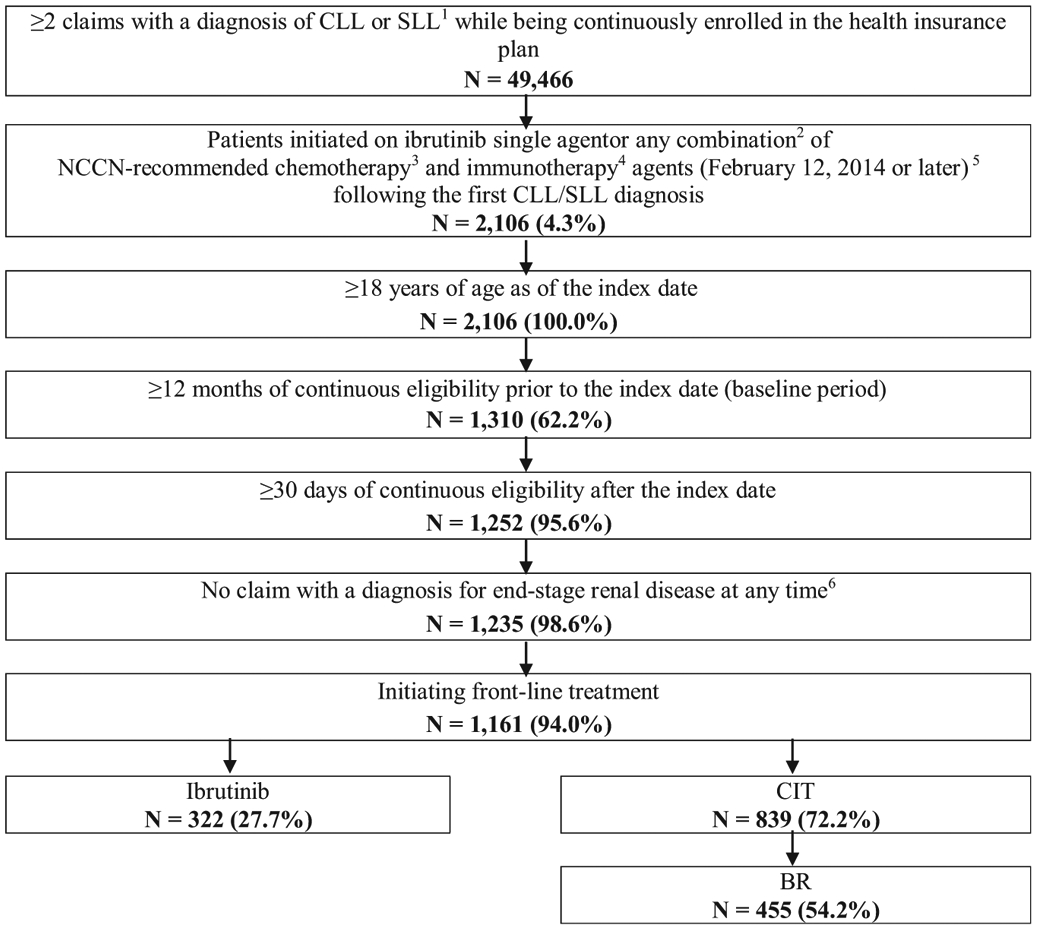
Abbreviations: BR = bendamustine/rituximab; CIT = chemoimmunotherapy; CLL = chronic lymphocytic leukemia; NCCN = National Comprehensive Cancer Network; SLL = small lymphocytic lymphoma. Notes: 1CLL diagnosis was identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) codes 204.1 and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10 CM) code C91.1. SLL diagnosis was identified using ICD-10 CM code C83.0. 2For combination therapy, the prescription date of each agent had to be within 30 days apart. 3Chemotherapy agents included bendamustine, chlorambucil, cyclophosphamide, cytarabine, doxorubicin, fludarabine, oxaliplatin, pentostatin, vincristine, lenalidomide, and cladribine. 4Immunotherapy agents included alemtuzumab, obinutuzumab, ofatumumab, and rituximab. 5The date of the first claim for one of these treatments following the first CLL/SLL diagnosis is the index date. 6End-stage renal disease was identified using ICD-9 CM code 585.6 and ICD-10 CM code N18.6.
