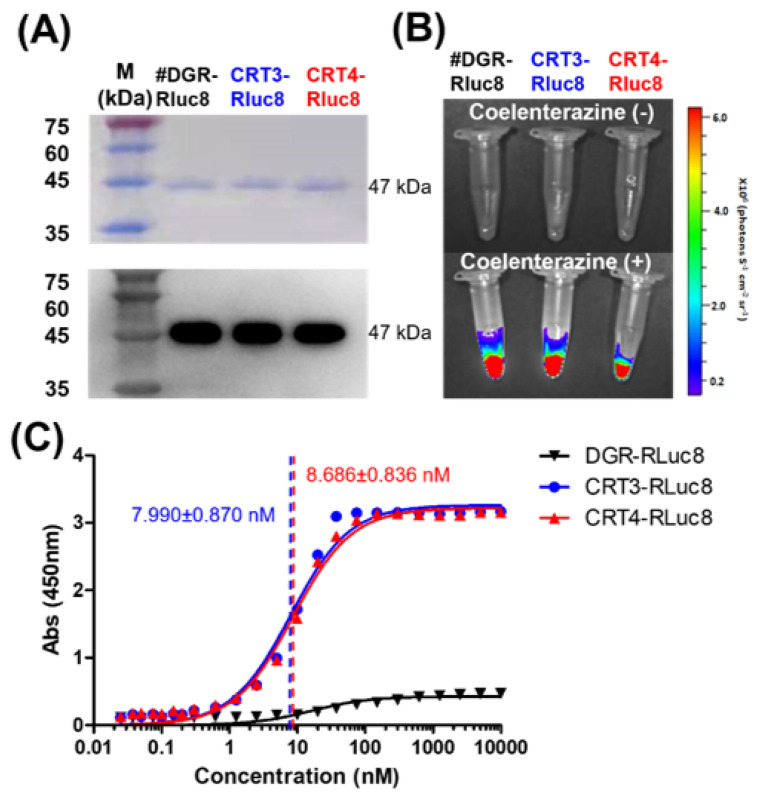
Figure 3.
Purification and affinity measurements of Rluc8-fused monobodies. (A) Purification of Rluc8-fused monobodies. The monobodies expressed in E. coli (47 kDa) were purified with affinity chromatography, separated by SDS-PAGE (stained with Coomassie dye), and verified by Western blot analysis (anti-His tag antibody). (B) Luciferase assay with Rluc8-fused monobodies. After adding coelenterazine, the bioluminescence of the monobodies was measured with an IVIS imaging system. (C) Binding affinity measurements of monobodies using enzyme-linked immunosorbent assay (ELISA). Various concentrations of Rluc8-fused monobodies (0–10 μM) were coated onto the wells of an ELISA plate and 10 μM rCRT was added to each well. rCRT bound to monobodies was detected with an anti-CRT antibody. The colorimetric reaction represents the amount of rCRT bound to monobodies. Absorbance values in were plotted against the monobody concentrations (logarithmic scale).