Abstract
Objectives
Previous research has documented a consistent association between current socioeconomic status (SES) and cytomegalovirus (CMV). Early life is likely a critical period for CMV exposure and immune development, but less is known about early-life socioeconomic factors and CMV, particularly in older age populations. Using data from the Health and Retirement Study, we investigated the association between life course socioeconomic disadvantage and immune response to CMV among older adults.
Methods
Using ordered logit models, we estimated associations between several measures of socioeconomic disadvantage and the odds of being in a higher CMV Immunoglobulin G (IgG) response category in a sample of 8,168 respondents aged older than 50 years.
Results
We found a significant association between educational attainment and CMV IgG response. Those with less than a high school education had 2.00 (95% confidence interval [CI]: 1.67–2.40) times the odds of being in a higher CMV category compared to those with a college degree or greater. In addition, we also observed a significant association with parental education and CMV response. Individuals with parents having 8 years or less of schooling had 2.32 (95% CI: 2.00–2.70) times the odds of higher CMV response compared to those whose parents had greater than high school education.
Discussion
CMV IgG levels in older adults are associated with both early-life and adult SES. Life course socioeconomic disadvantage may contribute to disparities in immunological aging.
Keywords: Immune aging, Life course studies, Persistent infections, Socioeconomic disadvantage
Disparities by social factors in age-related physical decline and disease onset have been widely described (Adler & Rehkopf, 2008; Adler & Stewart, 2010; Mensah et al., 2005). Immune dysfunction across the life course is a potential mechanism underlying these disparities (Roberts et al., 2010; Simanek et al., 2009). Specifically, exposure to infections during sensitive periods in early life may promote later chronic disease through early programming of immune and inflammatory responses (Dowd, Zajacova et al., 2009; McDade, 2005b; Meier et al., 2016). Existing evidence suggests that persistent infections such as cytomegalovirus (CMV) are associated with chronic diseases ranging from cancers to cardiovascular disease to cognitive decline and dementia (Aiello et al., 2006; Aiello, Chiu et al., 2017; Dowd, Zajacova et al., 2009; Liu et al., 2006; Patel et al., 1995; Schmaltz et al., 2005; Walboomers et al., 1999).
Numerous studies have suggested that CMV infection and immune response to CMV (as measured by Immunoglobulin G [IgG] antibody levels) may play a critical role in the process of immune aging and age-related declines in health overall, including all-cause mortality (Aiello et al., 2018; Aiello, Jayabalasingham et al., 2017; Feinstein et al., 2016; Pawelec et al., 2005; Simanek et al., 2011, 2015). CMV seropositivity and IgG antibody levels increase significantly with age (Fülöp et al., 2013; Wikby et al., 2005). However, studies have also found similar age-associated alterations in the immune compartment due to CMV in younger populations (Karrer et al., 2009; Weinberger et al., 2007), suggesting that higher CMV IgG antibody levels can be utilized as an indirect marker of lowered cell-mediated immunity, independent of age (Aiello & Dowd, 2012; Aiello et al., 2018). This is also supported by studies showing that CMV is associated with telomere shortening and telomerase activity, further suggesting CMV’s role in cellular aging (Aiello, Jayabalasingham et al., 2017; Dowd et al., 2017).
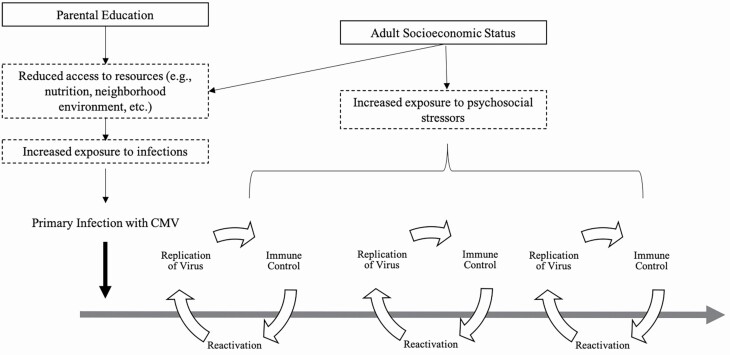
Primary infection with CMV often occurs early in life (Colugnati et al., 2007) and is followed by cycles of reactivation of the virus throughout the life course (Fülöp et al., 2013; Figure 1). Each cycle of reactivation requires substantial immune resources to force the virus into a quiescent state, effectively aging the immune system (Fülöp et al., 2013; Weinberger et al., 2007). Higher CMV IgG antibody levels among those who are seropositive, therefore, reflect either an initial response to primary infection or an impaired ability to keep CMV latent in the many cell types where it resides after the initial infection.
Figure 1.
A conceptual framework describing how socioeconomic status may be associated with cytomegalovirus (CMV) IgG response.
We draw upon life course theories of social status and health to conceptualize the link between life course social disadvantage and CMV response in late life (Figure 1), focusing both on sensitive period and cumulative disadvantage models. While a formal test of these theories is not possible given the available data, we nevertheless find that these models are helpful in framing the processes we believe may be at work. In the sensitive period model, early-life stress exposures may have lasting impacts on CMV response in late life because of reduced investment in the immune system in early life, particularly cell-mediated immunity which may have enduring consequences for immune aging across the life course (Barker, 1998; Ben-Shlomo & Kuh, 2002; McDade, 2005a). Such a model would predict that early-life social disadvantage would accelerate the pace of immune aging leading to poor immune control of CMV in late life, independent of adult disadvantages.
A cumulative disadvantage model predicts that early-life disadvantage is critical to adult health due to an accumulation of disadvantage across the life course—disadvantage in early life leads to increased risk of disadvantage at later stages, increasing disease risk at all life stages (Dannefer, 1997, 2003; Ferraro & Shippee, 2009; O’Rand, 1996). In the current study, disadvantage in early life would be reproduced across time, producing widening inequalities in both socioeconomic status (SES) and the pace of immune aging among older individuals (Crystal et al., 2017). Accordingly, those who experience multiple time points of socioeconomic disadvantage across the life course would have an accelerated pace of immune aging leading to poor immune control of CMV in late life. Therefore, we might expect experiences of more recent disadvantage to be more strongly associated with adult immune response to CMV than experiences of disadvantage in early life.
Moreover, it is likely that SES is fundamental both in determining the likelihood of initial exposure to and infection with CMV (McDade, 2005a, 2005b), as well as the frequency with which cycles of reactivation occur (Figure 1). We use parental education as a proxy measure of early-life social disadvantage. Individuals who grow up in households with low parental education have been shown to have an increased likelihood of poor housing conditions, less access to material resources, and neighborhood environments that limit access to health care compared with individuals who grow up in households with a higher level of parental education (Galobardes et al., 2007)—all of which could increase exposure to CMV. Studies among U.S. children have found that lower parental SES and minority race/ethnicity were associated with a higher prevalence of infections, including CMV (Dowd, Zajacova et al., 2009; Gares et al., 2017). This same pattern holds for adult SES in which lower SES in adulthood is associated with a higher prevalence of persistent infection with CMV and other persistent pathogens in both the United States and United Kingdom (Aiello et al., 2009; Dowd, Aiello et al., 2009; Simanek et al., 2009; Zajacova et al., 2009)
SES is also likely an important predictor of reactivation of CMV through the stress process. Social stress theory posits that individuals experiencing social disadvantage are differentially exposed to chronic stress (Pearlin, 1989), which may result in increased physiological wear and tear on several body systems (Juster et al., 2010), including the immune system. Indeed, stress accumulation throughout the life course is a key mechanism for explaining how cumulative inequality accelerates the aging process (Ferraro & Shippee, 2009). Thus, we posit that via chronic stress, social disadvantage may result in poorer immune control of CMV (i.e., more frequent cycles of reactivation of the virus). This would be evidenced by higher circulating IgG antibodies to CMV among those experiencing more disadvantage and particularly so among those who have experienced disadvantage at multiple time points across the life course (Glaser & Kiecolt-Glaser, 1994; Kuo et al., 2008; Rector et al., 2014; van Zanten et al., 1995). Indeed, studies have found that chronic stress is associated with increased inflammation (Friedman & Herd, 2010; Pollitt et al., 2008) and changes in immune function (Fagundes et al., 2012; Janicki-Deverts et al., 2014), specifically cell-mediated immune function. In addition, higher CMV IgG antibody levels have been observed with stressors such as academic testing (Glaser, 2005) and acute hospital care (Clari et al., 2013). Importantly, one recent study found that consistently experiencing disadvantage at multiple time points (i.e., cumulative disadvantage) across the life course was associated with higher IgG antibodies to CMV and other persistent pathogens in a population-based study of older Latinos (Meier et al., 2016).
Using a life course framework, we hypothesize that social disadvantage in both early- and late life will be associated with a poor immune response to CMV in late life. Though formal tests of life course theories are limited by the availability of longitudinal SES markers, we believe employing a life course framework can nonetheless provide a helpful lens through which to understand the processes at work. Furthermore, while existing studies suggest that SES across the life course may influence patterns of CMV infection and immune response critical for health in late-life, population-based studies on life course SES and later-life CMV infection in U.S. population-based studies of aging have been scarce. Using newly released biomarker data from the Health and Retirement Study (HRS), the current study investigates the association between life course socioeconomic disadvantage and CMV IgG antibodies among a nationally representative sample of aging adults.
Method
Study Population
Data come from the HRS, the largest ongoing nationally representative longitudinal survey of older adults in the United States. The HRS began in 1992 with more than 22,000 adults aged older than 50 years at baseline, with follow-up every 2 years. Additional details on HRS design and methods have been previously described (Juster & Suzman, 1995; Sonnega et al., 2014).
Our analysis used existing demographic and social data from the 1998, 2014, and 2016 waves, combined with the newly released immunological data from the 2016 Venous Blood Sample (VBS; Crimmins, 2017). A total of 20,912 individuals participated in the HRS 2016 survey, of whom 16,056 were panel respondents. All panel respondents in the 2016 interview were asked to participate in the VBS. The 2016 refresher sample (born between 1960 and 1965) were not eligible for the VBS. Of the panel participants, 61.9% consented to and participated in the VBS (n = 9,934). Our analysis included those who were deemed eligible based on the criteria below. Of the 9,934 participants, 7.5% were excluded based on having a survey weight of zero (n = 745). In HRS, weights of zero are assigned to deceased respondents, respondents residing in nursing homes, and those who are deemed age ineligible. In our sample, those with zero weights were deemed age ineligible, born after 1959. Another 6% were excluded due to missing data on parental education (n = 606). Those missing data on CMV were also excluded (0.7% of the sample, n = 74) and 1.3% were excluded because of missing data on other covariates (n = 139). Finally, we only included those categorized as non-Hispanic Black, Hispanic, or non-Hispanic White, yielding a final sample size of 8,168. Details of the construction of the study sample are shown in Supplementary Figure 3.
Measures
Adult SES was measured using participant education level and current household income. Education was categorized as less than high school, high school diploma or equivalent, some college, or college graduate and above (reference group). Household income was based on the continuous variable of household income to poverty ratio (IPR).
Childhood SES was measured using parental education. The highest level of education from either parent was used to construct parental education. The final parental education variable was a four-level variable comparing those with 8 years or less of education, those with 9–11 years of education, and those with a high school diploma to those with greater than high school education.
CMV seroprevalence and CMV IgG response were measured using IgG antibodies to CMV in serum using the Roche e411 immunoassay analyzer. The lower limit of detection was 0.015 antibody units per milliliter (U/mL). Results were reported as nonreactive (<0.5 antibody U/mL), borderline (0.5 to <1.0 antibody U/mL), or reactive (≥1.0 antibody U/mL). Those with a borderline response were classified as seropositive for CMV. For analytical purposes, we first examined seropositives (≥0.5 antibody U/mL) and seronegatives (<0.5 antibody U/mL). We then split the seropositives into tertiles of CMV antibody response using seronegatives as the reference group. The mean (standard error) CMV antibody response for seronegatives was 0.10 (0.0006) antibody U/mL. The mean (standard error) CMV antibody response for tertile 1 was 107.07 (2.10) antibody U/mL, 401.25 (3.01) for tertile 2, and 888.19 (8.86) for tertile 3.
Covariates included continuous age in years, gender (men/women), and race/ethnicity. Race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, or Hispanic.
Statistical Analyses
All statistical analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC). Descriptive statistics characterized the study population overall. Ordered logit models were used to estimate the relative odds of being in a higher category of CMV IgG response by adult SES (i.e., educational attainment and IPR) and childhood SES. Model 1 estimated the association of adult SES and CMV IgG levels controlling for age, gender, and race/ethnicity. Model 2 further adjusted for childhood SES by incorporating parental education, which we expected to be a life course predecessor of adult SES. Finally, Model 3 estimated the direct association of childhood SES and CMV IgG response, without adult SES. All analyses were weighted with the 2016 VBS weights.
Sensitivity Analyses
Several sensitivity analyses were performed. First, based on the different distribution and rewards of educational attainment by race/ethnicity for these cohorts (Fischer & Hout, 2006), we explored differences in the association between SES and CMV response by race/ethnic group. To do so, we included a two-way interaction term of educational attainment by race/ethnicity in a model including educational attainment, household IPR, age, gender, race/ethnicity, and parental education. We then reported the p value testing the statistical significance for the overall interaction term. We then explored race/ethnic-stratified models.
Next, we used a latent variable of childhood SES constructed by Vable et al. (2017) to conduct a sensitivity analysis incorporating multiple dimensions of the childhood socioeconomic environment to provide a more robust measure of childhood social disadvantage. Notably, the childhood SES index was only constructed for a subsample of HRS (N = 6,264), thus precluding us from using it in the primary analysis. Further details and all results are presented in Supplementary Material.
Results
Sample Characteristics
Overall, 65% of the sample was seropositive for CMV (Table 1). A greater proportion of non-Hispanic Blacks (32%) and those with less than a high school education (33%) were classified in the highest tertile of CMV IgG antibody response. This is contrasted with the percentage of non-Hispanic Whites and those with a college education or above who had 19% and 15%, respectively, classified in the highest tertile of CMV antibody response. The same pattern was observed for parental education as well where those with the lowest parental education levels were more likely to be in the highest tertile of CMV antibody response. Additionally, those with a lower mean household income to poverty level were more likely to be classified in the highest tertile of CMV antibody response. The complete demographic and socioeconomic characteristics of our study population are described in Table 1.
Table 1.
Weighted Distribution of Baseline Sociodemographic Characteristics of Eligible HRS Participants, Stratified by CMV Response Category (N = 8,168)
| Demographic characteristics | Total | % CMV Seronegative (35.29%) | % CMV Tertile 1 (22.04%) | % CMV Tertile 2 (21.51%) | % CMV Tertile 3 (21.09%) |
|---|---|---|---|---|---|
| Education | |||||
| Less than HS | 11.6 | 12.34 | 26.73 | 28.23 | 32.64 |
| HS graduate | 31.35 | 31.06 | 23.24 | 21.34 | 24.31 |
| Some college | 27.21 | 38.13 | 21.99 | 20.18 | 19.63 |
| College graduate | 29.84 | 46.06 | 19.03 | 20.28 | 14.57 |
| HH income to poverty ratio | |||||
| Mean HH IPR | 5.81 (0.12) | 6.74 (0.20) | 5.52 (0.26) | 5.79 (0.31) | 4.61 (0.17) |
| Parental education | |||||
| 8 years or less | 21.68 | 15.04 | 27.34 | 27.33 | 30.24 |
| 9–11 years | 12.98 | 22.16 | 25.03 | 25.16 | 27.50 |
| HS graduate | 37.44 | 41.77 | 20.38 | 19.39 | 18.44 |
| Greater than HS | 27.89 | 48.46 | 18.80 | 18.13 | 14.59 |
| Race/ethnicity | |||||
| Black | 9.48 | 11.55 | 27.91 | 28.23 | 32.30 |
| Hispanic | 8.57 | 7.09 | 31.48 | 31.16 | 30.18 |
| White | 81.95 | 40.99 | 20.39 | 19.73 | 18.85 |
| Age (years) | |||||
| Age | 66.59 (0.14) | 64.64 (0.20) | 67.86 (0.30) | 67.56 (0.29) | 67.55 (0.34) |
| Gender | |||||
| Female | 54.69 | 46.78 | 52.30 | 58.16 | 66.81 |
| Male | 45.31 | 53.20 | 47.70 | 41.8 | 33.1 |
Notes: CMV = cytomegalovirus; HH = household; HRS = Health and Retirement Study; HS = high school; IPR = income to poverty ratio. Weighted means and standard errors are reported for continuous variables. Weighted frequency distributions are reported for categorical variables. Higher CMV tertile indicates higher CMV IgG antibody response.
Ordered Logit Results
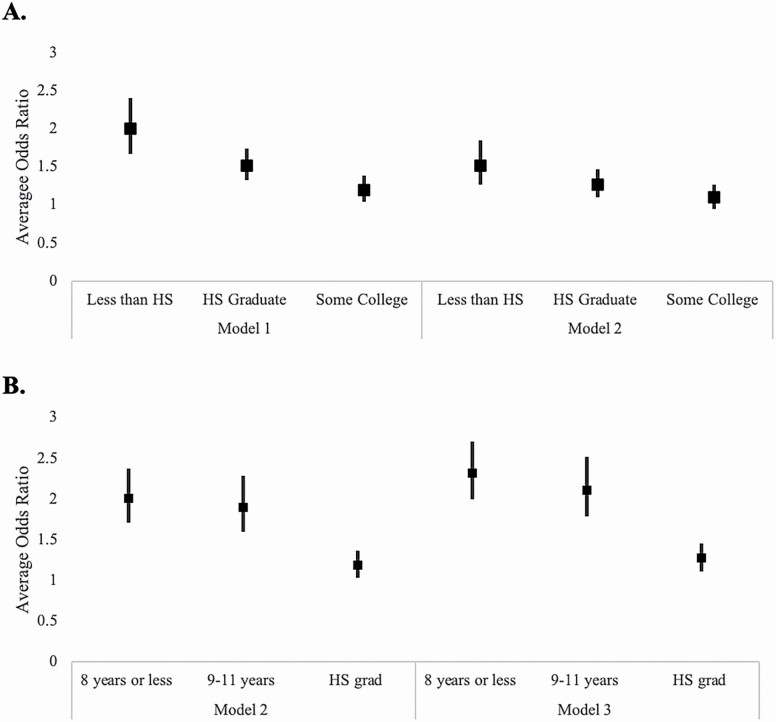
We found statistically significant associations between the level of educational attainment and CMV IgG response tertiles across models. In Model 1, we observed an educational gradient in CMV IgG response whereby those with less education had higher odds of being in a higher CMV response category compared to those with less education. For example, those with less than a high school education had 2.00 (95% confidence interval [CI]: 1.67–2.40) times the odds of being in a higher CMV response category compared to those with a college degree or greater (Model 1; Figure 2A). In models controlling for parental education, the association between participant educational attainment and CMV response was attenuated (Model 2). There were no statistically significant associations observed between household IPR and CMV response category.
Figure 2.
(A) Estimated relative odds ratio of being in the next sequentially higher cytomegalovirus (CMV) IgG response category by adult socioeconomic status (SES) in the Health and Retirement Study, Venous Blood Sample 2016. Note: Model 1 includes adult SES, race/ethnicity, age, gender, and marital status. Model 2 includes adult SES, race/ethnicity, age, gender, marital status, and parental education. Those with a college education or higher are the reference group. (B) Estimated relative odds ratio of being in the next sequentially higher CMV IgG response category by parental education in the Health and Retirement Study, Venous Blood Sample 2016. Note: Model 2 includes adult SES, race/ethnicity, age, gender, marital status, and parental education. Model 3 includes race/ethnicity, age, gender, marital status, and parental education. Having at least one parent with greater than a high school education is the reference group.
We next examined the association between childhood SES, as measured by parental education, and CMV IgG tertiles, both with and without adjustment for adult SES (Figure 2B). Across models, there was a significant association between parental education and CMV response that remained when controlling for adult SES (Models 2 and 3). Controlling for adult SES, individuals with parents with 8 years or less of schooling had 2.32 (95% CI: 2.00–2.70) times the odds of being in a higher CMV response category compared to those with parents having greater than a high school education (Model 3). Full results of the regression analyses are given in Table 2.
Table 2.
Ordered Logit Analyses Estimating the Odds of Being in the Next Sequentially Higher CMV IgG Response Category by Both Adult SES and Parental Education in the Health and Retirement Study, Venous Blood Sample 2016 (N = 8,168)
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Educational attainment | ||||||
| Less than HS education | 2.00 | 1.67–2.40 | 1.52 | 1.26–1.85 | ||
| HS education | 1.51 | 1.32–1.74 | 1.27 | 1.10–1.47 | ||
| Some college | 1.19 | 1.04–1.38 | 1.10 | 0.95–1.27 | ||
| College graduate or above (ref) | ||||||
| Household income to poverty ratio | 1.00 | 0.99–1.01 | 1.00 | 0.99–1.01 | ||
| Parental education | ||||||
| 8 years or less | 2.01 | 1.71–2.37 | 2.32 | 2.00–2.70 | ||
| 9–11 years | 1.90 | 1.59–2.28 | 2.11 | 1.78–2.52 | ||
| HS graduate | 1.18 | 1.03–1.36 | 1.27 | 1.11–1.45 | ||
| Greater than HS (ref) | ||||||
| Race | ||||||
| Non-Hispanic Black | 2.54 | 2.19–2.95 | 2.30 | 1.99–2.66 | 2.39 | 2.07–2.75 |
| Hispanic | 2.34 | 1.99–2.75 | 1.90 | 1.60–2.25 | 2.07 | 1.76–2.44 |
| Non-Hispanic White (ref) | ||||||
| Age (years) | 1.02 | 1.02–1.03 | 1.01 | 1.01–1.02 | 1.02 | 1.01–1.02 |
| Gender (female vs. male) | 1.63 | 1.47–1.81 | 1.63 | 1.47–1.81 | 1.65 | 1.49–1.83 |
Notes: CMV = cytomegalovirus; HS = high school; SES = socioeconomic status. Significant results at alpha = 0.05 are bolded. Model 1 includes race/ethnicity, adult SES, age, and gender. Model 2 includes race/ethnicity, adult SES, parental education, age, and gender. Those with a college education or higher are the reference group. Model 3 includes race/ethnicity, parental education, age, and gender. Having at least one parent with greater than a high school education is the reference group.
Sensitivity Analyses
In sensitivity analyses, we examined whether the association between SES and CMV response differed by race/ethnicity. First, we tested the statistical significance of a two-way interaction term of educational attainment × race/ethnicity in a model including education attainment, household IPR, age, gender, race/ethnicity, and parental education. The interaction term for the overall interaction was not statistically significant (p = .12). Nonetheless, given the substantial literature documenting differences in the burden of CMV by race/ethnicity, we examined race/ethnic-stratified models. Demographic characteristics stratified by race/ethnicity are presented in Supplementary Table 3. Among non-Hispanic Blacks and Hispanics, there were lower proportions of college education, lower household income, and lower levels of parental education compared to Whites. However, due to the small proportion of the sample who were non-Hispanic Black (9.48%) or Hispanic (8.57%), inferences regarding the association between SES and CMV response for each group were limited. For non-Hispanic Blacks, we only observed a significant association comparing those with less than a high school education to those with college or above and only in Model 1 (Supplementary Table 4). For non-Hispanic Whites, the association between educational attainment and CMV response followed a dose–response pattern across models. There was no significant association between educational attainment and CMV response for Hispanics in any of the models.
We next examined the association between childhood SES, as measured by parental education, and CMV IgG tertiles, both with and without adjustment for adult SES. For non-Hispanic Blacks, Hispanics, and non-Hispanic Whites, there was a significant association between parental education and CMV response that remained when controlling for adult SES, though this association was more consistently observed among non-Hispanic Whites. The race/ethnicity-stratified analyses suggest that the findings with regard to SES and CMV response are largely reflective of patterns among non-Hispanic Whites. However, conclusions are tempered by the small sample sizes of race/ethnic-stratified models.
We next replicated our models using the latent variable of childhood SES that incorporated multiple dimensions of childhood social capital, financial capital, and human capital elements. Notably, this variable was only available on a subset of the study population. We found similar associations whereby lower childhood SES was significantly associated with an increased odds of being in a higher CMV response category, net of adult SES (Supplementary Tables 5 and 6).
Discussion
Using a nationally representative aging cohort, we examined associations between life course SES and CMV IgG levels to better clarify the relationship between life course SES and immune aging. We found strong associations (odds ratios ranging from 1.52 to 2.00) between adult educational attainment and CMV IgG levels, but no association between adult income and CMV IgG. We also found evidence of both direct and indirect associations between childhood SES and CMV IgG levels. Our results suggest that early-life social status, including parental education and one’s own educational attainment, may shape the aging immune system. Overall, poor immune control may be one mechanism by which life course socioeconomic disadvantage contributes to disparities in age-related decline and early mortality.
Our results are consistent with existing studies documenting associations between low SES and both CMV seropositivity and CMV IgG levels, although we find differences depending on the measure of SES examined (Cannon et al., 2010; Feinstein et al., 2016). While we observed a significant association between adult educational attainment and CMV IgG levels, we did not observe a statistically significant association between household IPR and CMV IgG levels. This is consistent with a study among an older Hispanic population that reported a significant association between more years of education and lower CMV IgG levels, but no association between household income and CMV IgG when controlling for educational attainment (Dowd et al., 2008). Another study examining adults aged 25 years and older found that educational attainment was more strongly associated with CMV IgG levels for women, while for men income was a stronger predictor (Dowd & Aiello, 2009). It is likely that current income does not reflect life course SES as well as educational attainment in our largely retired cohort, and thus may not be as relevant a predictor of CMV level for older adults. Educational attainment is generally a more stable indicator of SES over the adult life span and reflects access to myriad resources such as income, neighborhood resources, and health-promoting behaviors, which likely explains its stronger association with CMV IgG in our sample (Link & Phelan, 1995; Zajacova & Lawrence, 2018).
The association between adult educational attainment and CMV response, net of parental education, suggests there may be a cumulative disadvantage process at work in which low adult educational attainment is a proxy of an underlying accumulation process of disadvantage. However, given that we were not able to employ longitudinal measures of social disadvantage and immune markers in the current study, a formal test of this theory was not feasible.
It may also be that the educational patterns are explained by considering the timing and duration of infection with CMV. Research has documented that socially disadvantaged populations (i.e., racial/ethnic minorities and/or low SES populations) are more likely to be infected with CMV at a younger age (Colugnati et al., 2007) and therefore live with the virus for a longer period of time. Thus, if an individual is already infected with CMV at an early age, the indirect protections afforded by educational attainment (i.e., reduced exposure to infection, living in less crowded conditions, and increased access to nutritious foods) may have less of an impact for these groups. The current findings support this hypothesis: We found strong independent associations between parental education and CMV response, net of participant educational attainment. This is also consistent with other studies examining the childhood social environment and CMV. In the nationally representative National Health and Nutrition Examination Survey (NHANES) III study, for example, children from low SES households and children of minority race/ethnicity status had a higher likelihood of CMV seroprevalence (Bate et al., 2010). However, Meier et al. (2016) found that the childhood environment, measured by parental education, occupation, and food availability as a child, was only significantly associated with adult CMV IgG levels through its influence on adult SES in a largely Latino sample 60–101 years old at baseline. Importantly, this would support a sensitive period model in which experiencing disadvantage in early life is an important predictor of late-life immune status, regardless of adult SES attainment. Future studies with improved social and environmental measures can help clarify the timing of these life course processes and their impact on both CMV seropositivity and CMV IgG levels.
In sensitivity analyses, we found some evidence that the association between educational attainment and CMV antibody response differed by race/ethnicity. However, these inferences were limited by the small proportion of the sample that was either non-Hispanic Black or Hispanic. Whereas Black participants had an overall higher predicted probability of CMV response than Whites, there was less of an educational gradient in CMV response among Blacks (i.e., higher education did not seem to confer the same magnitude of benefit with regard to CMV response among Blacks compared to Whites; Supplementary Table 4). A growing body of research has documented the differential returns to health at equal levels of education among racial/ethnic minority groups, particularly Blacks (Assari, 2018), and across health outcomes. Fundamental cause theory, for example, predicts that disadvantaged race/ethnic groups may realize fewer economic and health benefits from equivalent levels of education due to constrained resources and opportunities (Link & Phelan, 1996). Furthermore, prior research also suggests that different baseline levels of exposures (e.g., quality education) and outcomes (e.g., higher baseline levels of CMV) across minority groups are central to understanding these patterns (Fuller-Rowell et al., 2015; Gaydosh et al., 2018; Hayward et al., 2015; Ward et al., 2019). For example, educational attainment was lower among Blacks for the HRS cohorts, and differences in educational quality were substantial for cohorts raised in the Jim Crow era (Fischer & Hout, 2006). Our findings support these hypotheses: Blacks had a higher predicted probability of CMV compared to Whites but there was not a consistent association between educational attainment and CMV response for Blacks in contrast to Whites. These findings suggest that there is another force driving the patterns we see in CMV response among racial/ethnic minorities. Future studies would benefit from examining the association between SES and CMV in cohorts with larger proportions of racial/ethnic minority populations.
More broadly speaking, higher overall pathogen burden (i.e., infection with multiple persistent pathogens such as CMV) has been proposed as one mechanism linking SES to accelerated biological aging overall (Aiello et al., 2009; Zajacova et al., 2009). A recent study reported higher pathogen burden, measured as a latent variable accounting for both number and combinations of several persistent pathogens, was associated with a significant increase in the respondent’s number of preclinical deficits (a measure of accelerated biological aging; Noppert et al., 2018). Moreover, analyses using the Whitehall II Heart Scan Study found that herpesvirus coinfections were associated with significant declines in leukocyte telomere length prospectively for a period of 3 years, and that CMV seemed to be the most important driver of these declines (Dowd et al., 2017). These studies highlight the potential role of persistent pathogens in the wear and tear of multiple biological systems, leading to disparities in age-related disease.
While we believe that this study offers important insights into the relationship between SES and CMV across the life course, it is not without limitations. The data on CMV were only collected at one point in time in HRS, and thus examining how CMV IgG levels change over the life course is not yet possible. Additionally, there could be misclassification of individuals as seronegative or seropositive, but our use of ordered categorical IgG levels should mitigate any bias resulting from such misclassification. While we believe our use of a life course framework is an important contribution of this work, the measure of parental education is based on self-reports from the respondents much later in life and thus may suffer from recall bias. As with most observational studies, the association of social factors with CMV cannot be interpreted as causal and is best interpreted as a description of the social distribution of this important marker of immunological aging, which likely arises from a variety of life course mechanisms. Finally, while CMV IgG levels are a commonly used proxy for immune function, future studies would benefit from the inclusion of additional phenotypic and function immune markers, such as T-cell surface markers, stimulation, and proliferation endpoints.
In conclusion, this study contributes to the growing body of literature showing the association between social disadvantage and biological processes occurring across the life course. In particular, our findings highlight a link between social disadvantage across the life course and an important biomarker related to immunity. Given that aging of the immune system is a critical predictor of age-related decline and disease development, our results suggest that social disadvantage may influence the pace of these processes. There have been several efforts focused on developing a vaccination for CMV (Schleiss, 2016), which could reduce the transmission of CMV in the total population. However, in order for vaccination efforts to affect the socioeconomic disparities observed in CMV infection, critical attention would need to be paid to the equitable distribution of such a vaccine. Among older age race/ethnic minorities, CMV antibody response may be a reflection of both earlier age at infection and altered immune response to CMV from differential burden of life course stressors (Williams et al., 1997). To that end, interventions that target upstream life course socioeconomic determinants of immunity and infection will likely have the largest impact on age-related changes in immunity. Therefore, it will be critical to continue to consider the importance of life course social resources for improving health in older age, in combination with ongoing clinical vaccine interventions for preventing CMV infection.
Supplementary Material
Acknowledgments
We are grateful to the Carolina Population Center for training support (T32 HD091058 to A. E. Aiello and R. A. Hummer) and for general support (P2C HD050924).
Funding
G. A. Noppert received salary support from the National Institute on Aging grant AG000029-41 and K99AG062749 and from the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant T32 HD091058.
Conflict of Interest
None declared.
Author Contributions
Consent to submit has been received explicitly from all coauthors. Authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
References
- Adler, N E, & Rehkopf, D H. (2008). US disparities in health: Descriptions, causes, and mechanisms. Annual Review of Public Health, 29, 235–252. doi: 10.1145/annurev.pubhealth.29.020907.090852 [DOI] [PubMed] [Google Scholar]
- Adler, N E, & Stewart, J. (2010). Health disparities across the lifespan: Meaning, methods, and mechanisms. Annals of the New York Academy of Sciences, 1186, 5–23. doi: 10.1111/j.1749-6632.2009.05337.x [DOI] [PubMed] [Google Scholar]
- Aiello, A E, & Dowd, J. (2012). Socio-economic status and immunosenescence. In Bosch J, Phillips A, & Lord J (Eds.), Immunosenescence: Psychosocial and behavioral determinants (pp. 433). Springer. doi: 10.1007/978-1-4614-4776-4_9 [DOI] [Google Scholar]
- Aiello, A E, Chiu, Y L, & Frasca, D. (2017). How does cytomegalovirus factor into diseases of aging and vaccine responses, and by what mechanisms? Geroscience, 39(3), 261–271. doi: 10.1007/s11357-017-9983-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello, A E, Diez-Roux, A, Noone, A M, Ranjit, N, Cushman, M, Tsai, M Y, & Szklo, M. (2009). Socioeconomic and psychosocial gradients in cardiovascular pathogen burden and immune response: The multi-ethnic study of atherosclerosis. Brain, Behavior, and Immunity, 23(5), 663–671. doi: 10.1016/j.bbi.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello, A E, Haan, M, Blythe, L, Moore, K, Gonzalez, J M, & Jagust, W. (2006). The influence of latent viral infection on rate of cognitive decline over 4 years. Journal of the American Geriatrics Society, 54(7), 1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x [DOI] [PubMed] [Google Scholar]
- Aiello, A E, Jayabalasingham, B, Simanek, A M, Diez-Roux, A, Feinstein, L, Meier, H C S, Needham, B. L., & Dowd, J B. (2017). The impact of pathogen burden on leukocyte telomere length in the Multi-Ethnic Study of Atherosclerosis. Epidemiology and Infection, 145(14), 3076–3084. doi: 10.1017/S0950268817001881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello, A E, Simanek, A, Stebbins, R, & Dowd, J. (2018). Infectious disease. In Kivimaki M, Batty G, Steptoe A, & Kawachi I (Eds.), Routledge international handbook psychosocial epidemiology (pp. 281–300). Routledge. doi: 10.4324/9781315673097 [DOI] [Google Scholar]
- Assari, S. (2018). Unequal gain of equal resources across racial groups. International Journal of Health Policy and Management, 7(1), 1–9. doi: 10.15171/ijhpm.2017.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, D J. (1998). In utero programming of chronic disease. Clinical Science (London, England: 1979), 95(2), 115–128. doi: 10.1042/cs19980019 [DOI] [PubMed] [Google Scholar]
- Bate, S L, Dollard, S C, & Cannon, M J. (2010). Cytomegalovirus seroprevalence in the United States: The national health and nutrition examination surveys, 1988–2004. Clinical Infectious Diseases, 50(11), 1439–1447. doi: 10.1086/652438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo, Y, & Kuh, D. (2002). A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. Oxford University Press. doi: 10.1093/ije/31.2.285 [DOI] [PubMed] [Google Scholar]
- Cannon, M J, Schmid, D S, & Hyde, T B. (2010). Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Reviews in Medical Virology, 20(4), 202–213. doi: 10.1002/rmv.655 [DOI] [PubMed] [Google Scholar]
- Clari, M A, Aguilar, G, Benet, I, Belda, J, Giménez, E, Bravo, D, Carbonell, J A, Henao, L, & Navarro, D. (2013). Evaluation of cytomegalovirus (CMV)-specific T-cell immunity for the assessment of the risk of active CMV infection in non-immunosuppressed surgical and trauma intensive care unit patients. Journal of Medical Virology, 85(10), 1802–1810. doi: 10.1002/jmv.23621 [DOI] [PubMed] [Google Scholar]
- Colugnati, F A, Staras, S A, Dollard, S C, & Cannon, M J. (2007). Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infectious Diseases, 7, 71. doi: 10.1186/1471-2334-7-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins, E, Faul, J, Thyagarajan, B, & Weir, D. (2017). Venous blood collection and assay protocol in the 2016 Health and Retirement Study. Retrieved from https://hrs.isr.umich.edu/sites/default/files/biblio/2016%20VBS%20data%20ver%207_2.pdf
- Crystal, S, Shea, D G, & Reyes, A M. (2017). Cumulative advantage, cumulative disadvantage, and evolving patterns of late-life inequality. The Gerontologist, 57(5), 910–920. doi: 10.1093/geront/gnw056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannefer, D. (1997). Inequality and old age. Ageing and Society, 17, 232. doi: 10.1017/s0144686x96246380 [DOI] [Google Scholar]
- Dannefer, D. (2003). Cumulative advantage/disadvantage and the life course: Cross-fertilizing age and social science theory. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 58(6), 327–337. doi: 10.1093/geronb/58.6.s327 [DOI] [PubMed] [Google Scholar]
- Dowd, J B, & Aiello, A E. (2009). Socioeconomic differentials in immune response. Epidemiology (Cambridge, Mass.), 20(6), 902–908. doi: 10.1097/EDE.0b013e3181bb5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd, J B, Aiello, A E, & Alley, D E. (2009). Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiology and Infection, 137(1), 58–65. doi: 10.1017/S0950268808000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd, J B, Bosch, J A, Steptoe, A, Jayabalasingham, B, Lin, J, Yolken, R, & Aiello, A E. (2017). Persistent herpesvirus infections and telomere attrition over 3 years in the Whitehall II cohort. The Journal of Infectious Diseases, 216(5), 565–572. doi: 10.1093/infdis/jix255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd, J B, Haan, M N, Blythe, L, Moore, K, & Aiello, A E. (2008). Socioeconomic gradients in immune response to latent infection. American Journal of Epidemiology, 167(1), 112–120. doi: 10.1093/aje/kwm247 [DOI] [PubMed] [Google Scholar]
- Dowd, J B, Zajacova, A, & Aiello, A. (2009). Early origins of health disparities: Burden of infection, health, and socioeconomic status in US children. Social Science & Medicine, 68(4), 699–707. doi: 10.1016/j.socscimed.2008.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes, C P, Bennett, J M, Alfano, C M, Glaser, R, Povoski, S P, Lipari, A M,Agnese, D M, Yee, L D, Carson, W E3rd, Farrar, W B, Malarkey, W B, Chen, M, & Kiecolt-Glaser, J K. (2012). Social support and socioeconomic status interact to predict Epstein-Barr virus latency in women awaiting diagnosis or newly diagnosed with breast cancer. Health Psychology, 31(1), 11–19. doi: 10.1037/a0025599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein, L, Douglas, C E, Stebbins, R C, Pawelec, G, Simanek, A M, & Aiello, A E. (2016). Does cytomegalovirus infection contribute to socioeconomic disparities in all-cause mortality? Mechanisms of Ageing and Development, 158, 53–61. doi: 10.1016/j.mad.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro, K F, & Shippee, T P. (2009). Aging and cumulative inequality: How does inequality get under the skin? The Gerontologist, 49(3), 333–343. doi: 10.1093/geront/gnp034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, C S, & Hout, M. (2006). Century of difference: How America changed in the last one hundred years. Russell Sage Foundation. doi: 10.1093/esr/jcr084 [DOI] [Google Scholar]
- Friedman, E M, & Herd, P. (2010). Income, education, and inflammation: Differential associations in a national probability sample (The MIDUS study). Psychosomatic Medicine, 72(3), 290–300. doi: 10.1097/PSY.0b013e3181cfe4c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Rowell, T E, Curtis, D S, Doan, S N, & Coe, C L. (2015). Racial disparities in the health benefits of educational attainment: A study of inflammatory trajectories among African American and white adults. Psychosomatic Medicine, 77(1), 33–40. doi: 10.1097/PSY.0000000000000128 [DOI] [PubMed] [Google Scholar]
- Fülöp, T, Larbi, A, & Pawelec, G. (2013). Human T cell aging and the impact of persistent viral infections. Frontiers in Immunology, 4, 271. doi: 10.3389/fimmu.2013.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galobardes, B, Lynch, J, & Smith, G D. (2007). Measuring socioeconomic position in health research. British Medical Bulletin, 81–82, 21–37. doi: 10.1093/bmb/ldm001 [DOI] [PubMed] [Google Scholar]
- Gares, V, Panico, L, Castagne, R, Delpierre, C, & Kelly-Irving, M. (2017). The role of the early social environment on Epstein Barr virus infection: A prospective observational design using the Millennium Cohort Study. Epidemiology and Infection, 145(16), 3405–3412. doi: 10.1017/S0950268817002515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaydosh, L, Schorpp, K M, Chen, E, Miller, G E, & Harris, K M. (2018). College completion predicts lower depression but higher metabolic syndrome among disadvantaged minorities in young adulthood. Proceedings of the National Academy of Sciences of the United States of America, 115(1), 109–114. doi: 10.1073/pnas.1714616114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser, R. (2005). Stress-associated immune dysregulation and its importance for human health: A personal history of psychoneuroimmunology. Brain, Behavior, and Immunity, 19(1), 3–11. doi: 10.1016/j.bbi.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Glaser, R, & Kiecolt-Glaser, J. (1994). Stress-associated immune modulation and its implications for reactivation of latent herpesviruses. Infectious Disease and Therapy Series, 13, 245–245. doi: 10.1016/s0002-9343(98)00160-0 [DOI] [Google Scholar]
- Hayward, M D, Hummer, R A, & Sasson, I. (2015). Trends and group differences in the association between educational attainment and US adult mortality: Implications for understanding education’s causal influence. Social Science & Medicine, 127, 8–18. doi: 10.1016/j.socscimed.2014.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki-Deverts, D, Cohen, S, Doyle, W J, Marsland, A L, & Bosch, J. (2014). Childhood environments and cytomegalovirus serostatus and reactivation in adults. Brain, Behavior, and Immunity, 40, 174–181. doi: 10.1016/j.bbi.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster, F T, & Suzman, R. (1995). An overview of the Health and Retirement Study. Journal of Human Resources, 30, S7–S56. doi: 10.2307/146277 [DOI] [Google Scholar]
- Juster, R P, McEwen, B S, & Lupien, S J. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews, 35(1), 2–16. doi: 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Karrer, U, Mekker, A, Wanke, K, Tchang, V, & Haeberli, L. (2009). Cytomegalovirus and immune senescence: Culprit or innocent bystander? Experimental Gerontology, 44(11), 689–694. doi: 10.1016/j.exger.2009.09.003 [DOI] [PubMed] [Google Scholar]
- Kuo, C P, Wu, C L, Ho, H T, Chen, C G, Liu, S I, & Lu, Y T. (2008). Detection of cytomegalovirus reactivation in cancer patients receiving chemotherapy. Clinical Microbiology and Infection, 14(3), 221–227. doi: 10.1111/j.1469-0691.2007.01895.x [DOI] [PubMed] [Google Scholar]
- Link, B G, & Phelan, J C. (1995). Social conditions as fundamental causes of disease. Journal of Health and Social Behavior, 80–94. 10.2307/2626958 [DOI] [PubMed] [Google Scholar]
- Link, B G, & Phelan, J C. (1996). Understanding sociodemographic differences in health—The role of fundamental social causes. American Journal of Public Health, 86(4), 471–473. doi: 10.2105/ajph.86.4.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, R, Moroi, M, Yamamoto, M, Kubota, T, Ono, T, Funatsu, A, Komatsu, H, Tsuji, T, Hara, H, Hara, H, Nakamura, M, Hirai, H, & Yamaguchi, T. (2006). Presence and severity of Chlamydia pneumoniae and Cytomegalovirus infection in coronary plaques are associated with acute coronary syndromes. International Heart Journal, 47(4), 511–519. doi: 10.1536/ihj.47.511 [DOI] [PubMed] [Google Scholar]
- McDade, T W (2005a). The ecologies of human immune function. Annual Review of Anthropology, 34, 495–521. doi: 10.1146/annurev.antrho.34.081804.120348 [DOI] [Google Scholar]
- McDade, T W (2005b). Life history, maintenance, and the early origins of immune function. American Journal of Human Biology, 17(1), 81–94. doi: 10.1002/ajhb.20095 [DOI] [PubMed] [Google Scholar]
- Meier, H C S, Haan, M N, Mendes de Leon, C F, Simanek, A M, Dowd, J B, & Aiello, A E. (2016). Early life socioeconomic position and immune response to persistent infections among elderly Latinos. Social Science & Medicine (1982), 166, 77–85. doi: 10.1016/j.socscimed.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah, G A, Mokdad, A H, Ford, E S, Greenlund, K J, & Croft, J B. (2005). State of disparities in cardiovascular health in the United States. Circulation, 111(10), 1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04 [DOI] [PubMed] [Google Scholar]
- Noppert, G A, Aiello, A E, O’Rand, A M, & Cohen, H J. (2018). Investigating pathogen burden in relation to a cumulative deficits index in a representative sample of US adults. Epidemiology and Infection, 146(15), 1968–1976. doi: 10.1017/S095026881800153X [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rand, A M. (1996). The precious and the precocious: Understanding cumulative disadvantage and cumulative advantage over the life course. The Gerontologist, 36(2), 230–238. doi: 10.1093/geront/36.2.230 [DOI] [PubMed] [Google Scholar]
- Patel, P, Mendall, M A, Carrington, D, Strachan, D P, Leatham, E, Molineaux, N, Levy, J, Blakeston, C, Seymour, C A, & Camm, A J. (1995). Association of Helicobacter pylori and Chlamydia pneumoniae infections with coronary heart disease and cardiovascular risk factors. BMJ (Clinical Research ed.), 311(7007), 711–714. doi: 10.1136/bmj.311.7007.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec, G, Akbar, A, Caruso, C, Solana, R, Grubeck-Loebenstein, B, & Wikby, A. (2005). Human immunosenescence: Is it infectious? Immunological Reviews, 205, 257–268. doi: 10.1111/j.0105-2896.2005.00271.x [DOI] [PubMed] [Google Scholar]
- Pearlin, L I. (1989). The sociological study of stress. Journal of Health and Social Behavior, 30(3), 241–256. [PubMed] [Google Scholar]
- Pollitt, R A, Kaufman, J S, Rose, K M, Diez-Roux, A V, Zeng, D, & Heiss, G. (2008). Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. Journal of Epidemiology and Community Health, 62(6), 484–491. doi: 10.1136/jech.2006.054106 [DOI] [PubMed] [Google Scholar]
- Rector, J L, Dowd, J B, Loerbroks, A, Burns, V E, Moss, P A, Jarczok, M N, Stalder, T, Hoffman, K, Fischer, J E, & Bosch, J A. (2014). Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain, Behavior, and Immunity, 38, 133–141. doi: 10.1016/j.bbi.2014.01.012 [DOI] [PubMed] [Google Scholar]
- Roberts, E T, Haan, M N, Dowd, J B, & Aiello, A E. (2010). Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. American Journal of Epidemiology, 172(4), 363–371. doi: 10.1093/aje/kwq177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiss, M R. (2016). Cytomegalovirus vaccines under clinical development. Journal of Virus Eradication, 2(4), 198–207. [PMC free article] [PubMed] [Google Scholar]
- Schmaltz, H N, Fried, L P, Xue, Q L, Walston, J, Leng, S X, & Semba, R D. (2005). Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. Journal of the American Geriatrics Society, 53(5), 747–754. doi: 10.1111/j.1532-5415.2005.53250.x [DOI] [PubMed] [Google Scholar]
- Simanek, A M, Dowd, J B, & Aiello, A E. (2009). Persistent pathogens linking socioeconomic position and cardiovascular disease in the US. International Journal of Epidemiology, 38(3), 775–787. doi: 10.1093/ije/dyn273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanek, A M, Dowd, J B, Pawelec, G, Melzer, D, Dutta, A, & Aiello, A E. (2011). Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One, 6(2), e16103. doi: 10.1371/journal.pone.0016103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanek, A M, Dowd, J B, Zajacova, A, & Aiello, A. (2015). Unpacking the ‘black box’ of total pathogen burden: Is number or type of pathogens most predictive of all-cause mortality in the United States? Epidemiology & Infection, 143(12), 2624–2634. doi: 10.1017/s0950268814003215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnega, A, Faul, J D, Ofstedal, M B, Langa, K M, Phillips, J W, & Weir, D R. (2014). Cohort profile: The Health and Retirement Study (HRS). International Journal of Epidemiology, 43(2), 576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vable, A M, Gilsanz, P, Nguyen, T T, Kawachi, I, & Glymour, M M. (2017). Validation of a theoretically motivated approach to measuring childhood socioeconomic circumstances in the Health and Retirement Study. PLoS One, 12(10), e0185898. doi: 10.1371/journal.pone.0185898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers, J M, Jacobs, M V, Manos, M M, Bosch, F X, Kummer, J A, Shah, K V, Snijders, P J, Peto, J, Meijer, C J, & Muñoz, N. (1999). Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of Pathology, 189(1), 12–19. doi: [DOI] [PubMed] [Google Scholar]
- Ward, J B, Gartner, D R, Keyes, K M, Fliss, M D, McClure, E S, & Robinson, W R. (2019). How do we assess a racial disparity in health? Distribution, interaction, and interpretation in epidemiological studies. Annals of Epidemiology, 29, 1–7. doi: 10.1016/j.annepidem.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger, B, Lazuardi, L, Weiskirchner, I, Keller, M, Neuner, C, Fischer, K H, Neuman, B, Würzner, R, & Grubeck-Loebenstein, B. (2007). Healthy aging and latent infection with CMV lead to distinct changes in CD8+ and CD4+ T-cell subsets in the elderly. Human Immunology, 68(2), 86–90. doi: 10.1016/j.humimm.2006.10.019 [DOI] [PubMed] [Google Scholar]
- Wikby, A, Ferguson, F, Forsey, R, Thompson, J, Strindhall, J, Löfgren, S, Nilsson, B O, Ernerudh, J, Pawelec, G, & Johansson, B. (2005). An immune risk phenotype, cognitive impairment, and survival in very late life: Impact of allostatic load in Swedish octogenarian and nonagenarian humans. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 60(5), 556–565. doi: 10.1093/gerona/60.5.556 [DOI] [PubMed] [Google Scholar]
- Williams, D R, Yu, Y, Jackson, J S, & Anderson, N B. (1997). Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology, 2(3), 335–351. doi: 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- Zajacova, A, Dowd, J B, & Aiello, A E. (2009). Socioeconomic and race/ethnic patterns in persistent infection burden among U.S. adults. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 64(2), 272–279. doi: 10.1093/gerona/gln012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajacova, A, & Lawrence, E M. (2018). The relationship between education and health: Reducing disparities through a contextual approach. Annual Review of Public Health, 39, 273–289. doi: 10.1146/annurev-publhealth-031816-044628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zanten, J, Harmsen, M C, van der Giessen, M, van der Bij, W, Prop, J, & De Leij, L (1995). Humoral immune response against human cytomegalovirus (HCMV)-specific proteins after HCMV infection in lung transplantation as detected with recombinant and naturally occurring proteins. Clinical and Diagnostic Laboratory Immunology, 2(2), 214–218. doi: 10.1128/cdli.2.2.214-218.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.