Abstract
Objectives:
To examine the burden of depressive symptoms across the adult age span in people with multiple sclerosis (MS) and test if the relationship between depressive symptoms and MS characteristics vary across age groups.
Methods:
In analyses of the MS Partners Advancing Technology and Health Solutions (MS PATHS) network of adults with MS, we compared the prevalence of depression in MS PATHS with non-MS controls across age and evaluated for effect modification by age in the association between depressive symptoms and clinical and neuroperformance measures via multivariable-adjusted regression models.
Results:
13,821 individuals with MS were included. The prevalence of depression was higher in MS versus non-MS controls, but was similar between men/women across age. The association between depression and processing speed (PST; p for interaction=0.009) or walking speed (p for interaction=0.04) varied by age. For example, younger depressed individuals had 0.45 SD (95% CI: −0.62, −0.29) worse PST Z-scores versus non-depressed younger participants, whereas older depressed individuals had 0.20 SD (95% CI: −0.32, −0.08) worse PST Z-scores versus non-depressed older participants.
Conclusions:
Depressive symptoms and age should be considered when interpreting measures of walking speed and cognitive function; these findings may have implications for analyses of neuroperformance change.
Keywords: depression, epidemiology, aging
INTRODUCTION
Multiple sclerosis (MS) is a central nervous system disorder characterized by inflammation, demyelination and neurodegeneration. Neuropsychiatric symptoms are reported in up to 60% of patients with MS and have been associated with lower medication adherence and quality of life [1]. Specifically, depression is common; the lifetime prevalence of depression in people with MS is estimated to reach 50% [2].
Older adults with MS experience greater disability and greater limitations in their activities of daily living and are more likely to experience a progressive disease course compared to their younger counterparts [3]. While findings on the relationship between age and change in neuropsychiatric symptomatology have been mixed [4]–[10], neuropsychiatric comorbidities [11] and suicidal ideation [12] remain prevalent in older adults with MS. Furthermore, prevalence studies have reported that depressive symptoms are undertreated in MS [8],[13], particularly in patients over 44 years of age [11].
Many factors have been examined in relation to neuropsychiatric symptoms across age groups in MS with inconsistent results, including disability [6],[14], changes in cognitive attitudes [9],[15], immunotherapy status [16], and disease status [17]. However, many of these studies included relatively small sample sizes or largely homogenous patient populations, which may limit generalizability, prevent detailed assessment of changes in depressive symptoms across the lifespan or preclude analyses of understudied MS subgroups of interest (e.g. different racial/ethnic groups). Thus, in a large, diverse population of over 13,000 people with MS, we compare the burden of depressive symptoms across the adult age span as well as evaluate if age modifies the association between depressive symptoms and participant characteristics or clinical measures of MS severity.
METHODS
Design
We conducted a cross-sectional study of a large cohort of people with MS in the United States and Europe in which clinical, neuroperformance and depressive symptoms were assessed from 2016 to 2020.
Study Population and Clinical Assessments
Data for these analyses were derived from the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS) system. The MS PATHS is a collaborative network of 7 centers in the United States and 3 centers in Europe sponsored by Biogen [18]. Institutional Review Board approval was granted by all US sites including Johns Hopkins University School of Medicine. Patients with confirmed MS at least 18 years of age were enrolled after providing informed consent. During routine clinical visits, participants complete the MS Performance Test (MSPT) [19]–[21], an iPad-based electronic adaptation of the MS Functional Composite (MSFC) consisting of assessments of walking speed (WST), manual dexterity (MDT) and processing speed (PST) combined with a detailed health questionnaire and quality of life assessment. Therein, participants report demographic (e.g. age, sex, ethnicity, race) and MS characteristics (age at symptom onset, disease subtype, disease modifying therapy). Neuroperformance MSPT outcomes were transformed into Z-scores using regression-based equations derived from a healthy volunteer study of individuals aged 18 to 89 (n=517) who completed a similar battery of assessments.[22] Each Z-scored neuroperformance outcome has a mean equal to 0 and standard deviation equal to 1 in the healthy controls; mean differences can be thus interpreted as a per SD difference in a given outcome. Clinical information including assessment of height and weight (used to calculate body mass index [BMI] as kg/m2), systolic and diastolic blood pressure, and concomitant medications (including antidepressants) were automatically abstracted from the electronic medical record (EMR). We identified antidepressant users as those taking a Serotonin-Norepinephrine Reuptake Inhibitor (SNRI; including venlafaxine, duloxetine, bupropion, mirtazapine, trazodone), Selective Serotonin Reuptake Inhibitor (SSRI; including paroxetine, fluoxetine, sertraline, citalopram, escitalopram), or other antidepressant medications (nefazodone, hydrochloride, rasagiline, vilazodone, milnacipran, fluvoxamine, vortioxetine). Radiology report data are also extracted from the EMR, including the number of T1 post-Gd enhancing lesions (if a post-contrast scan was acquired; 0–3, >3) and the number of new T2 lesions since the prior MRI (if a comparable scan was available; 0–3, >3), as read by the local radiologists at each institution.
Assessment of Depressive Symptoms and Other Quality of Life Domains
The MSPT also includes Quality of Life in Neurological Disorders (Neuro-QoL) subscales to measure quality of life in 12 domains including depression, fatigue, cognitive function, sleep disturbance, ability to participate in social roles and activities, and satisfaction with social roles and activities [23]. Raw Neuro-QoL raw scores were converted to T-scores calibrated to a general reference population for analysis; a T-score of 50 represents the mean of a measure within its reference population, with a standard deviation of 10. For depressive symptoms, the reference population used to calculate is a clinical population of individuals with neurological disorders, and higher Neuro-QoL T- scores denote more severe symptoms. For other MS QoL domains of interest (fatigue, cognitive function, sleep disturbance), T-scores were derived similarly. For fatigue and sleep disturbance, higher scores denote more severe fatigue or sleep disturbance; for cognitive function, lower scores denote more severe self-reported cognitive impairment.
Statistical Analysis
Initial analyses used descriptive summary statistics and univariate methods to describe demographic, clinical, QoL and medication (including antidepressants and medication class) characteristics of the cohort across categories of depressive symptoms (none, mild, moderate, severe). We used Neuro-QoL suggested T-score cut-points to categorize depressive symptoms as normal [T-score ≤ (mean + 0.5SD)), mild [(mean + 0.5SD) < T-score ≤ mean + 1SD)], moderate [(mean + 1SD) < T-score ≤ (mean + 2SD)], and severe [T-score > (mean + 2SD)]. We compared clinical, comorbidity, and disease characteristics across subtypes using univariate generalized linear regression models, as appropriate. We also compared the prevalence of moderate-to-severe depression in MS PATHS with the prevalence of moderate-to-severe depression in the National Health and Nutrition Examination Survey (NHANES) survey respondents from 2017–2018 across age groups (<30 years old [yo], 30–39yo, 40–49yo, 50–59yo, ≥60yo). We compared the prevalence of antidepressant use in MS PATHS and NHANES across similar age categories. NHANES is a representative sample of the US population, designed to characterize the health status of people living in the US items of the study. We defined moderate to severe depressive symptoms in MS PATHS as having a Neuro-QoL T-score ≥ 1SD above the mean. Depressive symptoms in NHANES are assessed using the Patient Health Questionnaire-9 (PHQ-9); moderate to severe depression in NHANES was defined using a score ≥10, an established cut-point for the PHQ-9 [24]. All prevalence estimates in NHANES data incorporated survey weights to account for the complex sampling procedure.
The prevalence of depression across racial groups and by sex may vary with age in people without MS [25]–[27]. Thus, we evaluated if age modifies the association between sex (women, men) and race (Black/African American, white, other) and depressive symptom severity in people with MS. To do so, we fit linear models for NeuroQoL depressive symptoms incorporating an interaction between age (considered as a continuous variable and categorized as <30 years old [yo], 30–39yo, 40–49yo, 50–59yo, ≥60yo) and race or sex. Formal tests of effect modification used likelihood ratio tests. All models were additionally adjusted for ethnicity, insurance status and years of education (as proxies for socio-economic status), insurance status, smoking status, BMI, disease duration, antidepressant use and disease modifying therapy (DMT) class; effect modification models for sex also adjusted for race and vice-versa. We also tested for potential effect modification by age on the association between depressive symptoms and clinical characteristics (e.g. MS subtype and DMT class) using a similar approach and adjusting for a similar set of relevant covariates.
Lastly, previous studies have suggested depression can negatively impact neuroperformance [28],[29]; however, whether this association is variable in different age groups is not well-described. We assessed for potential effect modification by age in models evaluating whether moderate-to-severe depression is associated with MSPT measures of neurologic function (WST, PST, MDT) using linear models regressing neuroperformance versus moderate-to-severe depression incorporating a cross-product between age and depressive symptom status. We used Z-scored values of MSPT measures and adjusted for a similar set of covariates as above. Similar multivariable-adjusted models evaluated effect modification by age in the association between depressive symptoms and common MS symptoms including sleep disturbance, fatigue, and subjective cognitive impairment. Formal tests of effect modification (for MSPT outcomes and for MS symptoms) used likelihood ratio tests.
RESULTS
Overall Characteristics of Study Participants
We included 13,821 MS PATHS participants with baseline NeuroQoL measures for depressive symptoms; 8817 (63.7%) reported no depressive symptoms, 2487 (18.0%) reported mild, 1672 (12.1%) reported moderate, and 845 (6.1%) reported severe depressive symptoms (Table 1). Notably, severe depressive symptoms were more common in Black or African American (AA) participants relative to White or Caucasian (CA) participants; 10.5% of AA report depressive symptoms while 5.5% of CA report severe depressive symptoms (p<0.001). Hispanic or Latinos also report more severe symptoms (e.g., 9.5% of Hispanic/Latinos report severe symptoms whereas 5.8% of non-Hispanic/Latinos or 5.4% of CA report severe symptoms). While age and sex were statistically associated with depressive symptom severity, the absolute difference across categories was small. Across categories of none, mild, moderate, and severe depressive symptoms, the mean ages were 48.91, 49.60, 48.90 and 46.17, respectively. The percentage of men in each category were 27.3%, 24.6%, 24.8%, and 26.0%. Individuals who were current smokers, had fewer years of education, had either no insurance or Medicaid insurance, and those who were unemployed or on disability were more likely to report more severe depressive symptoms (all p <0.001). Prescribed antidepressants were relatively common in MS PATHS (4226 [30.6%] participants; Supplemental Table 1). The prevalence of antidepressant use increased with depression severity (p<0.001). Of those prescribed antidepressants, 2231 (16.2%) were prescribed SSRIs, 1920 (13.9%) were prescribed SNRIs and 69 (0.5%) were prescribed other antidepressants; sertraline was the most commonly prescribed SSRI and duloxetine was the most commonly prescribed SNRI. The burden of depressive symptoms was more severe for those prescribed antidepressants; 10.9% of those prescribed antidepressants report severe symptoms, while 4.0% without an antidepressant prescription report severe symptoms.
Table 1.
Descriptive Characteristics of included MS PATHS participants by categories of depressive symptoms
| None | Mild | Moderate | Severe | P-value* | |
|---|---|---|---|---|---|
| N | 8817 (63.7) | 2487 (18.0) | 1672 (12.1) | 845 (6.1) | |
|
| |||||
| NeuroQoL depression T-scores, mean (SD) | 42.14 (5.09) | 51.44 (1.10) | 56.14 (1.62) | 63.47 (3.35) | <0.001 |
| Use of antidepressant, n (%) | 2131 (24.2) | 908 (36.5) | 725 (43.4) | 462 (54.7) | <0.001 |
| Age, mean (SD), years | 48.91 (12.47) | 49.60 (12.12) | 48.90 (12.23) | 46.17 (11.90) | <0.001 |
| Male sex, n (%) | 2406 (27.3) | 613 (24.6) | 414 (24.8) | 220 (26.0) | 0.02 |
| Race, n (%) | <0.001 | ||||
| White or Caucasian | 7121 (80.8) | 1952 (78.5) | 1246 (74.5) | 596 (70.5) | |
| Black or African American | 695 (7.9) | 210 (8.4) | 166 (9.9) | 126 (14.9) | |
| Other | 483 (5.5) | 154 (6.2) | 145 (8.7) | 73 (8.6) | |
| Unknown | 518 (5.9) | 171 (6.9) | 115 (6.9) | 50 (5.9) | |
| Hispanic or Latino ethnicity, n (%) | 303 (3.4) | 85 (3.4) | 88 (5.3) | 50 (5.9) | <0.001 |
| Years of education, mean (SD) | 14.67 (3.07) | 13.99 (3.06) | 13.59 (3.08) | 13.29 (3.16) | <0.001 |
| Insurance status, n (%) | <0.001 | ||||
| Private, state | 7252 (82.3) | 1838 (73.9) | 1128 (67.5) | 474 (56.1) | |
| Medicare | 1077 (12.2) | 408 (16.4) | 329 (19.7) | 188 (22.2) | |
| Medicaid or none | 429 (4.9) | 220 (8.8) | 195 (11.7) | 179 (21.2) | |
| Unknown | 59 (0.7) | 21 (0.8) | 20 (1.2) | 4 (0.5) | |
| Current smoker, n (%) | 1047 (11.9) | 412 (16.6) | 364 (21.8) | 208 (24.6) | <0.001 |
| Employment status, n (%) | <0.001 | ||||
| Full time/student | 5372 (61.2) | 1099 (44.5) | 596 (35.9) | 224 (26.5) | |
| Part time | 926 (10.6) | 250 (10.1) | 180 (10.8) | 76 (9.0) | |
| Retired | 959 (10.9) | 336 (13.6) | 187 (11.3) | 61 (7.2) | |
| Disabled | 1106 (12.6) | 593 (24.0) | 504 (30.3) | 346 (41.0) | |
| Unemployed | 267 (3.0) | 134 (5.4) | 149 (9.0) | 113 (13.4) | |
| Other | 147 (1.7) | 58 (2.3) | 46 (2.8) | 24 (2.8) | |
| Body mass index, mean (SD), kg/m2 | 28.49 (6.72) | 29.35 (7.33) | 29.30 (7.40) | 30.02 (7.70) | <0.001 |
| MS subtype, n (%) | |||||
| Relapsing-remitting / CIS | 5983 (67.9) | 1413 (56.8) | 834 (49.9) | 359 (42.5) | <0.001 |
| Progressive | 2015 (22.9) | 833 (33.5) | 681 (40.7) | 419 (49.6) | |
| Unknown | 819 (9.3) | 241 (9.7) | 157 (9.4) | 67 (7.9) | |
| Age at symptom onset, mean (SD) | 32.68 (11.03) | 32.73 (11.69) | 31.73 (11.67) | 30.24 (11.09) | <0.001 |
| Disease duration, mean (SD) | 14.24 (10.88) | 14.92 (11.13) | 15.09 (11.08) | 14.04 (10.62) | 0.003 |
| Disease modifying therapy, n (%) | <0.001 | ||||
| Injectable | 2031 (23.0) | 519 (20.9) | 284 (17.0) | 134 (15.9) | |
| Oral | 2519 (28.6) | 665 (26.7) | 431 (25.8) | 197 (23.3) | |
| Infusion | 1589 (18.0) | 481 (19.3) | 345 (20.6) | 209 (24.7) | |
| None | 1756 (19.9) | 523 (21.0) | 417 (24.9) | 202 (23.9) | |
| Other | 517 (5.9) | 178 (7.2) | 116 (6.9) | 70 (8.3) | |
P-values are derived from a t-tests or Chi-square tests for a given variable and depressive symptom severity.
Evaluation of Effect Modification by Age in the Association between Participant Characteristics and Depressive Symptoms
A. Overall Depressive Symptom Burden by Age Group in MS PATHS and NHANES
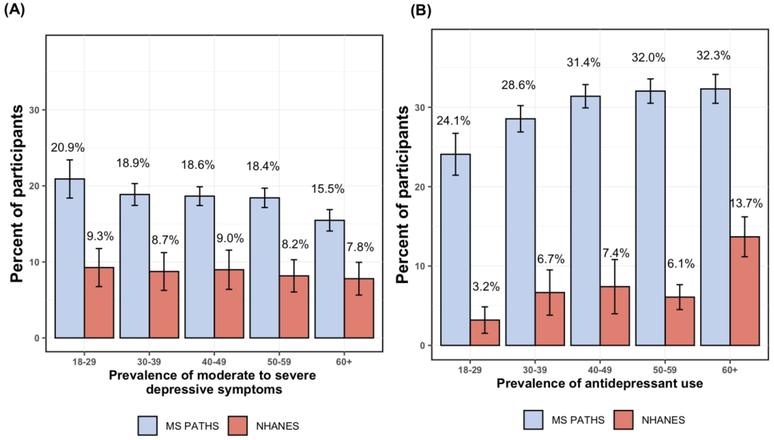
The prevalence of moderate-to-severe depressive symptoms was higher in the MS population (18.2%) relative to NHANES (8.5%), regardless of age (Figure 1). This difference in prevalence was relatively consistent across all age groups. A similar pattern was observed for prescribed antidepressants, with antidepressant use being more common in MS PATHS than NHANES across all age groups. Yet, within each cohort, the burden of moderate-to-severe depression slightly decreased with increasing age in both MS PATHS (20.9% 18–29yo’s to 15.3% in 60+yo’s) and NHANES participants (9.3% in 18–29yo’s to 7.9% in 60+yo’s). In MS PATHS, the prevalence of antidepressant prescriptions increased from 24.1% in 18–29yo’s to 32.3% in 60+yo’s. In NHANES, the prevalence of antidepressant prescriptions increased from 3.2% in 18–29yo’s to 13.4% in 60+yo’s.
Figure 1. Prevalence of moderate to severe depressive symptoms by age in MS PATHS and NHANES (A) and the prevalence of any antidepressant use in MS PATHS and NHANES (B).
B. Analyses of Effect Modification by Age in the Association between Depressive Symptoms and Participant Characteristics
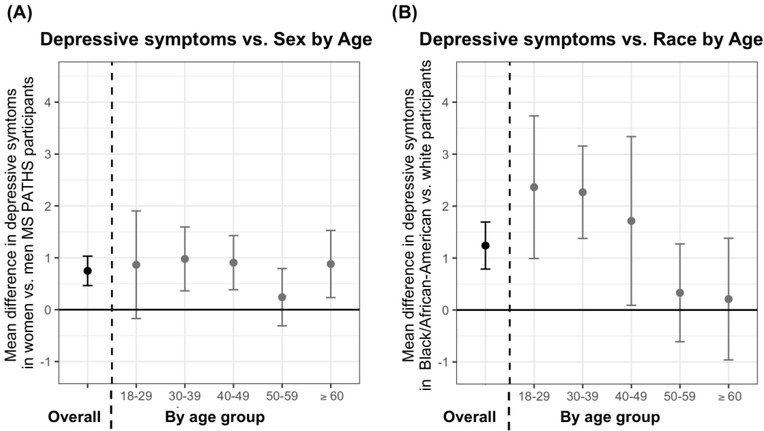
The association between sex and depressive symptom severity did not vary by age (p for interaction=0.22; Figure 2). However, we observed an effect modification by age in the association between race and depressive symptom severity (p for interaction<0.001; Figure 2). Relative to CA of the same age group, AA 18–29yo’s had 2.73 points (95% CI: 1.20, 4.23) higher Neuro-QoL depressive symptom T-score. In contrast, the burden of depressive symptoms did not differ between AA and CA 60+yo participants (mean difference: 0.05; 95% CI: −1.04, 1.14). Since the underlying burden of inflammation could vary between AA and CA and across age groups, we also performed analyses adjusting for new lesions in the previous year (n=6568 with MRI) and restricting to participants taking higher efficacy therapies (n=2624); results were consistent in both sets of analyses, with younger AA participants having higher depressive symptoms relative to CA participants of the same age group. Age did not modify the association between clinical characteristics including MS subtype or DMT class (all p for interaction > 0.05).
Figure 2. Depressive symptom severity versus selected participant characters by age.
(A) The association between depressive symptom severity sex adjusted for race, ethnicity, education status, insurance, disease duration, DMTs class, antidepressant use, smoking status, and BMI. Estimates and error bars denote the difference in depressive symptoms between women vs. men overall (black; left of dotted line) and by age groups (gray; right of dotted line). The graph demonstrates that women users overall have an average of 0.75 points (95% CI: 0.47, 1.03) higher NeuroQoL depression scores relative to men. This association was similar across age groups (p=0.22). (B) The association between depressive symptom severity race adjusted for a similar set of confounders, as above. Estimates and error bars denote the difference in depressive symptoms between Black/African American (AA) vs. white (CA) participants overall (black; left of dotted line) and by age groups (gray; right of dotted line). We detected significant effect modification by age (p=0.0007). For example, AA 18–29yo’s report an average of 2.73 points higher NeuroQoL depression scores relative to CA 18–29yo’s (adjusted mean difference in Neuro-QoL scores: 2.73; 95% CI: 1.20, 4.23). In contrast, there was no difference in depressive symptom severity between AA versus CA 60+yo’s (adjusted mean difference: 0.05; 95% CI: −1.04, 1.14).
C. Analyses of Effect Modification by Age in the Association between Neurologic Function and Depressive Symptoms.
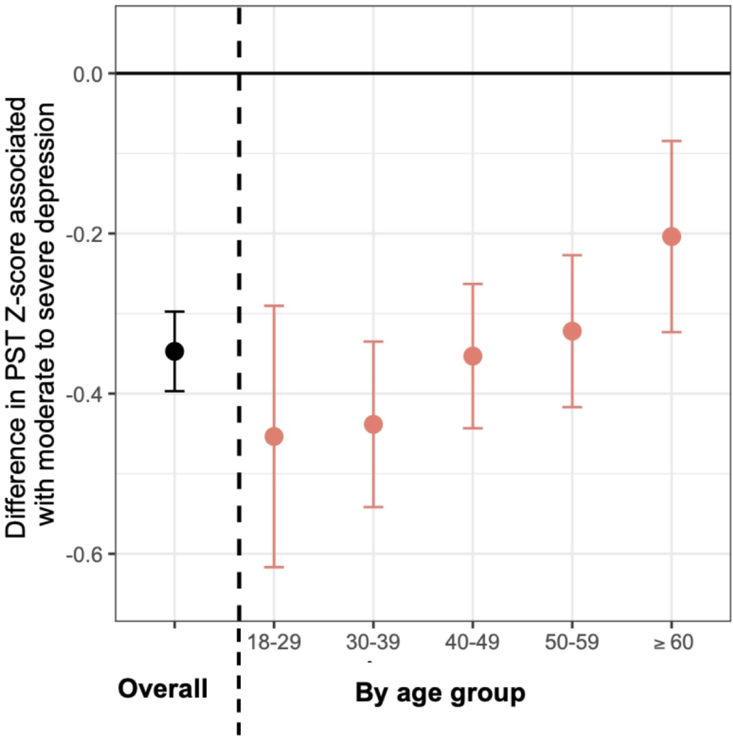
Age modified the association between depressive symptoms and objective measures of neurological function including WST and PST Z-scores in different ways (Table 2; Figure 3). With respect to measures of processing speed, younger individuals with moderate-to-severe depressive symptoms had worse PST Z-scores (p for interaction=0.009). For example, 18–29yo’s with moderate-to-severe symptoms had 0.45 SD worse PST Z-scores relative to non-depressed 18–29yo’s (−0.45 SD; 95% CI: −0.62, −0.29). In comparison, in 60+yo’s, the strength of the association was reduced by >50%; moderate-to-severely depressed 60+yo’s had 0.20 SD worse PST Z-scores relative to non-depressed 60+yo’s (−0.20 SD; 95% CI: −0.32, −0.08). With respect to measures of ambulation, younger individuals with moderate-to-severe depressive symptoms had less impaired WST Z-scores relative to non-depressed individuals in the same age group (p for interaction=0.04); 18–29yo’s with moderate-to-severe symptoms did not have significantly worse WST Z-scores relative to non-depressed 18–29yo’s (0.00 SD; 95% CI: −0.59, −0.59). In comparison, moderate-to-severely depressed 60+yo’s had 0.56 SD worse WST Z-scores relative to non-depressed individuals aged 60+yo’s (−0.56 SD; 95% CI: −0.99, −0.12). We did not detect effect modification by age in the association between depression and manual dexterity or other MS symptoms like fatigue or sleep disturbance (all p for interaction > 0.05).
Table 2.
Evaluation of effect modification by age in the association between moderate-to-severe depressive symptoms and neurological function
| Overall | Age category |
P for interaction | |||||
|---|---|---|---|---|---|---|---|
| 18 – 29 | 30 – 39 | 40 – 49 | 50 – 59 | 60 + | |||
|
| |||||||
| WST Z-score, mean difference* (95% CI) | −0.55 (−0.73, −0.37) | 0.00 (−0.59, 0.59) | −0.42 (−0.80, −0.05) | −0.60 (−0.93, −0.27) | −0.74 (−1.09, −0.40) | −0.56 (−0.99, −0.12) | 0.04 |
| PST Z-score, mean difference* (95% CI) | −0.35 (−0.40, −0.30) | −0.45 (−0.62, −0.29) | −0.44 (−0.54, −0.34) | −0.35 (−0.44, −0.26) | −0.32 (−0.42, −0.23) | −0.20 (−0.32, −0.30) | 0.009 |
| MDT Z-score, mean difference* (95% CI) | −0.50 (−0.58, −0.42) | −0.53 (−0.79, −0.29) | −0.56 (−0.73, −0.39) | −0.44 (−0.58, −0.29) | −0.50 (−0.66, −0.35) | −0.44 (−0.64, −0.24) | 0.62 |
Mean differences are additionally adjusted for race, ethnicity, education status, insurance, disease duration, DMTs class, antidepressant use, smoking status, and BMI.
Figure 3. Association Between Depression Severity and Processing Speed Z-score* by Age Group.
*Z-scores were calculated from regression-based equations derived from a healthy volunteer study of individuals aged 18 to 89 (n=517) of MSPT outcomes, including processing speed. The mean for each neuroperformance outcome is equal to 0 and the standard deviation is equal to 1 among the healthy controls. Values presented denote a per SD difference in a given outcome. Mean differences are adjusted for race, ethnicity, education status, insurance, disease duration, DMTs class, antidepressant use, smoking status, and BMI. Estimates and error bars denote the difference in PST Z-scores between moderate-to-severe vs. no depressive symptoms (black; left of dotted line) and by age groups (pink; right of dotted line). Individuals reporting moderate to severe depressive symptoms had −0.35 SD lower PST- Z scores relative to those with mild to no depressive symptoms. This association was modified by age (p = 0.009). For example, 18yo-30yo’s with moderate to severe depression had −0.45 SD worse PST Z-scores relative to non-depressed individuals aged 18yo to 30yo (−0.45 SD; 95% CI: −0.62, −0.29). In comparison, moderate-to-severely depressed 60+yo’s have −0.20 SD worse PST Z-scores relative to non-depressed 60+yo’s (−0.20 SD; 95% CI: −0.32, −0.08).
DISCUSSION
In these analyses, we describe the prevalence of depressive symptoms across the lifespan in nearly 14,000 people with MS. We confirm that people with MS have a higher burden of depressive symptoms relative to a comparable non-MS US population and also find that this difference does not vary across age groups. In contrast to patterns observed in the general population where females have a higher lifetime prevalence of mood disorders [30], female sex was not associated with higher prevalence of depressive symptoms in MS PATHS across age groups. Younger AA participants had a notably higher burden of symptoms relative to younger CA participants. We also note that depressive symptoms were generally associated with worse neuroperformance (as measured by walking speed, manual dexterity and processing speed), and that this association varied with age. In younger individuals, moderate-to-severe depressive symptoms were associated with slower processing speed while in older individuals it was associated with slower walking speed.
Our findings are consistent with previous studies examining the prevalence of self-reported psychiatric symptoms across different age groups in MS, which have reported a trend towards lower prevalence of depressive symptoms with increasing age. In a cross-sectional study comparing self-reported psychiatric symptoms across 4 age categories in 1282 adults with MS over 45 yo, the >75 yo and 64–75 yo age groups had the lowest prevalence of psychiatric symptoms (10% and 14%, respectively) [8]. A study utilizing data of 322 patients from the Canadian Community Health Survey reported the prevalence of major depression, as determined by the Composite International Diagnostic Interview Short Form for Major Depression (CIDI-SFMD) in 18–45 year olds to be 25.7%, while prevalence in >45 yo was 8.4% [15]. Depression in MS has been associated with inflammation [31], and the shift from inflammatory to neurodegenerative pathology with age has been proposed as an underlying mechanism for differences in depressive symptom prevalence across age groups [32]. While we did not find evidence that age modified the relationship between depression and MS subtype or DMT class, future studies accounting for MS disease severity and duration are needed to directly address this question.
Our observations that walking speed and processing speed are associated with depression is consistent with the prior literature. Studies directly examining walking speed and depression in MS are limited, though physical disability has been associated with depression in MS [2]. A study of 33 MS patients with depressive symptoms and 33 without found that those with depressive symptoms have slower processing speed compared to those without depression [28]. Another cross-sectional study of 721 patients found that self-reported depressive symptoms were associated with slower processing speed [29]. However, age was not examined as a covariate in these studies. Intriguingly, we found that the association between moderate-to-severe depression and objective measures of neuroperformance varied across the adult age span. For example, younger individuals with more severe depressive symptoms tended to perform worse on the PST when compared to older individuals with more severe depressive symptoms. For WST, higher depressive symptom burden was only associated with worse walking speed in older individuals. These findings are particularly notable as they suggest both age and depressive symptoms should be taken into account when interpreting these scores in both cross-sectional/single time-point analyses as well as in analyses of change in neuroperformance. Depressive symptom severity can be dynamic; estimates of the magnitude of change in PST or WST in the context of changing depressive symptom burden may differ depending on the age distribution of the population under study. One strength of the MS PATHS approach is that both depressive symptoms and neuroperformance are measured at each time a patient has an appointment, which will allow future studies to account for these potential differences in effect across age.
Consistent with prior MS PATHS analyses [33], we also observed that younger AA experience a higher burden of depressive symptoms than younger CA. This be explained in part by prior studies that have pointed to a more aggressive disease course in AA [34],[35] and underdiagnosis of depression in AA. A study comparing the odds of diagnosed depression and clinically significant depressive symptoms in MS found that AA had a nearly twofold increased odds of having undiagnosed major depression than CA [11]. The burden of underlying inflammation may also be higher in AA versus CA; increased inflammation may also contribute to depressive symptoms in some people [36]. Further studies are needed to further elucidate this finding and investigate other potential contributing underlying factors for these differences, including potential underlying socio-demographic determinants.
While improved case detection of depression may identify individuals who would benefit from treatment, further study is needed to understand the barriers to adequate management of depressive symptoms in MS. Though 30.6% of MS PATHS participants were on antidepressants, many of those participants (28%) continued to report moderate to severe depressive symptoms. These findings support previous reports that depression is undertreated in patients with comorbid MS and depression [13] in spite evidence for effective interventions. Most randomized controlled trials of psychotherapy have demonstrated efficacy in reducing depressive symptoms in MS [37],[38]. While open label trials have demonstrated that pharmacotherapy is effective in reducing depression symptoms in MS [37], conclusions from randomized controlled trials have varied based on the definition of efficacy and have been limited by the use of pharmacotherapies known to have grater adverse effect profiles [39].
This study has several strengths, including the inclusion of a large, well-defined cohort of individuals with MS where a broad array of demographic, clinical and functional characteristics are available and collected using validated instruments. Nevertheless, these findings should be interpreted in the context of its limitations. Symptoms of depression relied on self-report using the NeuroQoL scale rather than formal diagnosis of depression. The use of antidepressants for indications outside of depression (e.g. neuropathic pain, insomnia or fatigue) could not be ruled out. Furthermore, it was unknown whether individuals on antidepressants had received a full therapeutic trial at the time of depressive symptom assessment. Hence although 54.7% of participants with severe depressive symptoms were on antidepressants, this may not necessarily reflect treatment refractory depression. We also lacked assessment of clinician-adjudicated recent relapse. Since relapse rates vary with age and ongoing inflammation may contribute to depressive symptom severity, it will be important to ascertain whether the association is similar after accounting for recent relapse in the future. Lastly, cross-sectional analyses do not capture the dynamic nature of depressive states, which may vary according to different patient characteristics. We were not able to assess longitudinal associations between age, race, social factors, other MS disease characteristics or neuroperformance and changes in depressive symptoms.
In summary, depressive symptoms are common in MS, and identification of risk factors is important in improving our detection and management of this common, but undertreated comorbidity. Future studies examining depressive symptoms longitudinally may provide further insight into variables associated with changes in psychiatric symptoms over the lifespan, particularly as they relate to concomitant change in neuroperformance and risk for disability progression.
Supplementary Material
Acknowledgments
Study Funding:
KCF is supported by U.S. Department of Health and Human Services, National Institutes of Health National Institute of Mental Health K01-MH121582 and a Career Transition Fellowship from the National Multiple Sclerosis Society TA-1805-31136. MS PATHS is supported by Biogen. Dr. Pimentel Maldonado is supported by a Sylvia Lawry Fellowship from the NMSS.
Disclosures:
Drs. Chan, Pimentel Maldonado, and Fitzgerald and Ms. Tian have nothing to disclose.
Dr. Mowry has grants from Biogen and Genzyme, is site PI for studies sponsored by Biogen, has received free medication for a clinical trial from Teva, and receives royalties for editorial duties from UpToDate.
References
- 1.Murphy R, O’Donoghue S, Counihan T, et al. Neuropsychiatric syndromes of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017; 88(8):697–708. [DOI] [PubMed] [Google Scholar]
- 2.Patten SB, Marrie RA, Carta MG. Depression in multiple sclerosis. Int. Rev. Psychiatry 2017; 29(5):463–472. [DOI] [PubMed] [Google Scholar]
- 3.Sanai SA, Saini V, Benedict RH, et al. Aging and multiple sclerosis. Mult. Scler. J 2016; 22(6):717–725. [DOI] [PubMed] [Google Scholar]
- 4.Alsaadi T, El Hammasi K, Shahrour TM, et al. Prevalence of Depression and Anxiety among Patients with Multiple Sclerosis Attending the MS Clinic at Sheikh Khalifa Medical City, UAE: Cross-Sectional Study. Mult. Scler. Int 2015; 2015:487159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beal CC, Stuifbergen AK, Brown A. Depression in multiple sclerosis: a longitudinal analysis. Arch. Psychiatr. Nurs 2007; 21(4):181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chwastiak L, Ehde DM, Gibbons LE, et al. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am. J. Psychiatry 2002; 159(11):1862–1868. [DOI] [PubMed] [Google Scholar]
- 7.Figved N, Klevan G, Myhr KM, et al. Neuropsychiatric symptoms in patients with multiple sclerosis. Acta Psychiatr. Scand 2005; 112(6):463–468. [DOI] [PubMed] [Google Scholar]
- 8.Garcia J, Finlayson M. Mental health and mental health service use among people aged 45+ with multiple sclerosis. Can. J. Commun. Ment. Health 2009; 24(2):9–22. [DOI] [PubMed] [Google Scholar]
- 9.Kneebone I, Dunmore E, Evans E. Symptoms of depression in older adults with multiple sclerosis (MS): comparison with a matched sample of younger adults. Aging Ment. Health 2003; 7(3):182–185. [DOI] [PubMed] [Google Scholar]
- 10.Mohammadi K, Rahnama P, Montazeri A. Prevalence and risk factors for depression in women with multiple sclerosis: a study from Iran. Ann. Gen. Psychiatry 2015; 14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrie R, Horwitz R, Cutter G, et al. The burden of mental comorbidity in multiple sclerosis: frequent, underdiagnosed, and undertreated. Mult. Scler. J 2009; 15(3):385–392. [DOI] [PubMed] [Google Scholar]
- 12.Klewer J, Pohlau D, Nippert I, Kugler J, others. Problems reported by elderly patients with multiple sclerosis. J. Neurosci. Nurs 2001; 33(3):167. [DOI] [PubMed] [Google Scholar]
- 13.Feinstein A An examination of suicidal intent in patients with multiple sclerosis. Neurology. 2002; 59(5):674–678. [DOI] [PubMed] [Google Scholar]
- 14.Bogart KR. Disability identity predicts lower anxiety and depression in multiple sclerosis. Rehabil. Psychol 2015; 60(1):105. [DOI] [PubMed] [Google Scholar]
- 15.Patten SB, Beck CA, Williams JV, Barbui C, Metz L. Major depression in multiple sclerosis: a population-based perspective. Neurology. 2003; 61(11):1524–1527. [DOI] [PubMed] [Google Scholar]
- 16.Möller A, Wiedemann G, Rohde U, Backmund H, Sonntag A. Correlates of cognitive impairment and depressive mood disorder in multiple sclerosis. Acta Psychiatr. Scand 1994; 89(2):117–121. [DOI] [PubMed] [Google Scholar]
- 17.Lorefice L, Fenu G, Trincas G, et al. Progressive multiple sclerosis and mood disorders. Neurol. Sci 2015; 36(9):1625–1631. [DOI] [PubMed] [Google Scholar]
- 18.Mowry E, Bermel RA, Williams JR, et al. Harnessing Real-world Data to Inform Decision-making: Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). Front. Neurol 2020; 11. Available at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00632/abstract [Accessed June 2, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldassari LE, Nakamura K, Moss BP, et al. Technology-enabled comprehensive characterization of multiple sclerosis in clinical practice. Mult. Scler. Relat. Disord 2020; 38:101525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao SM, Galioto R, Sokolowski M, et al. Multiple Sclerosis Performance Test: validation of self-administered neuroperformance modules. Eur. J. Neurol 2020; 27(5):878–886. [DOI] [PubMed] [Google Scholar]
- 21.Rao SM, Galioto R, Sokolowski M, et al. Multiple Sclerosis Performance Test: validation of self-administered neuroperformance modules. Eur. J. Neurol 2020; 27(5):878–886. [DOI] [PubMed] [Google Scholar]
- 22.Sokolowski M, Dey T, Strober L, Banks S, Miller JB, Norman M, Gooding A, Reece C, Busson B, de Moor C, Williams JR, Rudick RA, Schindler D, Alberts JL, Rao SM. Multiple Sclerosis Performance Test (MSPT): A Demographically Stratified Normative Database (N = 517) of Healthy Subjects Ages 10–89 to Aid Clinical Interpretation of MS Patient Neuroperformance. 2019.
- 23.Miller DM, Bethoux F, Victorson D, et al. Validating Neuro-QoL Short Forms and Targeted Scales with Persons who have Multiple Sclerosis. Mult. Scler. Houndmills Basingstoke Engl 2016; 22(6):830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001; 16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bey GS, Waring ME, Jesdale BM, Person SD. Gendered race modification of the association between chronic stress and depression among Black and White U.S. adults. Am. J. Orthopsychiatry 2018; 88(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hargrove TW, Halpern CT, Gaydosh L, et al. Race/Ethnicity, Gender, and Trajectories of Depressive Symptoms Across Early- and Mid-Life Among the Add Health Cohort. J. Racial Ethn. Health Disparities 2020; 7(4):619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salk RH, Hyde JS, Abramson LY. Gender Differences in Depression in Representative National Samples: Meta-Analyses of Diagnoses and Symptoms. Psychol. Bull 2017; 143(8):783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubrini G, Periáñez JA, Fernández-Fournier M, et al. Identifying Perceptual, Motor, and Cognitive Components Contributing to Slowness of Information Processing in Multiple Sclerosis with and without Depressive Symptoms. Span. J. Psychol 2020; 23. Available at: https://www.cambridge.org/core/journals/spanish-journal-of-psychology/article/identifying-perceptual-motor-and-cognitive-components-contributing-to-slowness-of-information-processing-in-multiple-sclerosis-with-and-without-depressive-symptoms/B23B6145DF7F6F874895828B7620D621 [Accessed July 12, 2020]. [DOI] [PubMed] [Google Scholar]
- 29.Macaron G, Baldassari LE, Nakamura K, et al. Cognitive processing speed in multiple sclerosis clinical practice: association with patient-reported outcomes, employment and magnetic resonance imaging metrics. Eur. J. Neurol 2020; 27(7):1238–1249. [DOI] [PubMed] [Google Scholar]
- 30.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005; 62(6):593–602. [DOI] [PubMed] [Google Scholar]
- 31.Gold SM, Irwin MR. Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Immunol. Allergy Clin. North Am 2009; 29(2):309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musella A, Gentile A, Rizzo FR, et al. Interplay between age and neuroinflammation in multiple sclerosis: effects on motor and cognitive functions. Front. Aging Neurosci 2018; 10:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Tian F, Fitzgerald KC, et al. Socioeconomic status and race are correlated with affective symptoms in multiple sclerosis. Mult. Scler. Relat. Disord 2020; 41:102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivas-Rodríguez E, Amezcua L. Ethnic Considerations and Multiple Sclerosis Disease Variability in the United States. Neurol. Clin 2018; 36(1):151–162. [DOI] [PubMed] [Google Scholar]
- 35.Caldito NG, Saidha S, Sotirchos ES, et al. Brain and retinal atrophy in African-Americans versus Caucasian-Americans with multiple sclerosis: a longitudinal study. Brain J. Neurol 2018; 141(11):3115–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol 2016; 16(1):22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiest K, Walker J, Bernstein C, et al. Systematic review and meta-analysis of interventions for depression and anxiety in persons with multiple sclerosis. Mult. Scler. Relat. Disord 2016; 5:12–26. [DOI] [PubMed] [Google Scholar]
- 38.Thomas PW, Thomas S, Hillier C, Galvin K, Baker R. Psychological interventions for multiple sclerosis. Cochrane Database Syst. Rev 2006; (1):CD004431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch MW, Glazenborg A, Uyttenboogaart M, Mostert J, De Keyser J. Pharmacologic treatment of depression in multiple sclerosis. Cochrane Database Syst. Rev 2011; (2):CD007295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.