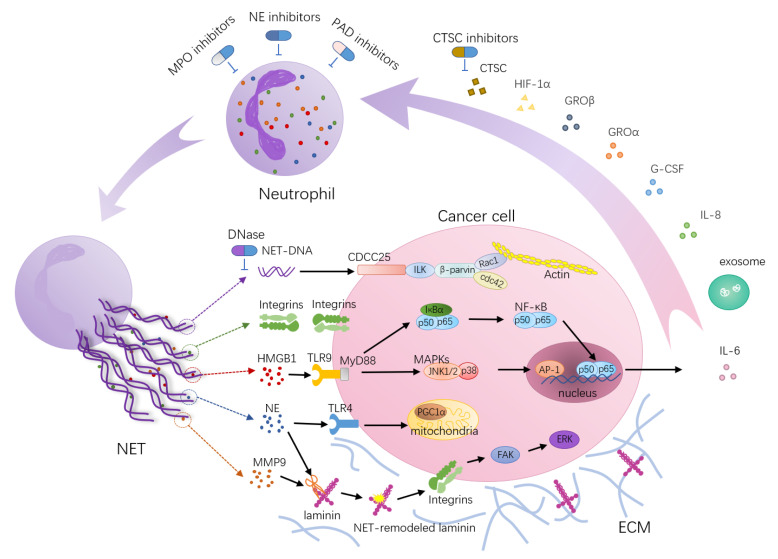
Figure 1.
Neutrophil extracellular trap (NET)-associated molecular mechanisms’ function in tumor metastasis. NETs act as scaffolds mediating the capture of cancer cells and providing a microenvironment that can bring protumorigenic proteins close to cancer cells. HMGB1, a highly conserved DNA binding protein, is one of the components of NETs. NETs trigger HMGB1 release and activate TLR9-dependent pathways in cancer cells. The tumorigenic effects of TLR9 depend on NF-κB-mediated upregulation of IL-6 expression and activation of a cascade of intracellular growth signaling pathways, including MAP kinase pathways. NE released from NETs activates TLR-4 on cancer cells, leading to PGC-1α upregulation, increased mitochondrial biogenesis and accelerated growth. NETs trap circulating tumor cells via β1-integrin-mediated interactions. NE and MMP-9 sequentially cut laminin, an important component of the ECM, revealing an epitope that triggers the proliferation of dormant cancer cells through integrin activation and FAK/ERK/MLCK/YAP signaling. Furthermore, the transmembrane protein CCDC25 is a NET DNA receptor on cancer cells that senses extracellular DNA and subsequently activates the ILK-β-parvin pathway to enhance cell motility. In turn, certain factors secreted by many primary tumors have been shown to promote NET formation, such as cytokines (HIF-1α, IL-8, G-CSF, GROα, GROβ), proteases (CTSCs) and exosomes. During these processes, targets for therapies have been postulated, and interfering drugs (blue arrows) have already been used in clinical practice or are under investigation in vivo.