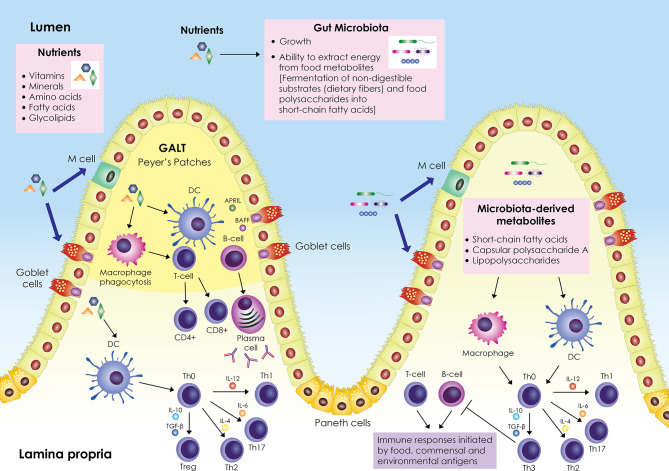
Figure 1.
Schematic representation of the interplay between nutrients, gut microbiota and immune system. The gut-associated lymphoid tissue (GALT) occupies a large area of the gut; it is scattered within the intestinal epithelium and is also organized into lymphoid follicles in the lamina propria called Peyer’s patches. GALT consists mainly of B and T cells, macrophages, and dendritic cells (DCs). Enterocytes (Paneth cells, goblet cells, microfold (M) cells) are responsible for the active transport or passive diffusion of antigens from food during digestion and microbial components. M cells, located in Peyer’s patches, take up luminal antigens by transcytosis and present them to underlying DCs in the lamina propria, which in turn interact with B and T cells either in Peyer’s patches or in mesenteric lymph nodes. DCs secrete cytokines and induce differentiation of T helper cell precursors (Th0) into effector Th cells (Th1, Th2, Th17) or Tregs. Antigen impingement on intestinal epithelial cells and subsequent activation of DCs induces differentiation of B cells into IgA-secreting plasma cells. In parallel, nutrients modulate the gut microbiota by promoting or inhibiting its growth and affecting its ability to derive energy from dietary chemicals. Microbiota-derived metabolites (short-chain fatty acids, capsular polysaccharide A, lipopolysaccharides) stimulate and modulate DCs and macrophages to create an antigen presentation environment that favors the differentiation of Th0 cells into Th3 Tregs that inhibit T- and B-cell inflammatory responses triggered by food, commensal, and environmental antigens. Adapted from refs (6–16).