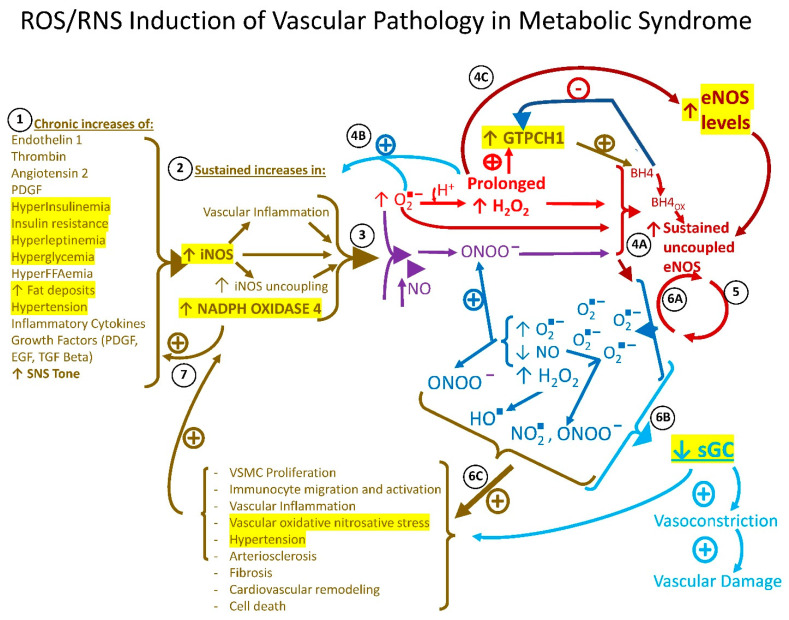
Figure 4.
ROS/RNS induction of vascular pathology in metabolic syndrome. Chronic increases in several humoral, neural, and metabolic factors of metabolic syndrome listed in 1 potentiate sustained increases in the expressions of NADPH oxidase 4 and iNOS. 2. Such increases in NADPH oxidase 4 and iNOS activities produce increases in oxygen free radical (O2−) that can react with H+ to form hydrogen peroxide (H2O2) and with nitric oxide (NO) to form peroxynitrite (ONOO−). 3. All three of O2−, H2O2, and ONOO− lead to eNOS uncoupling via oxidation of tetrahydrobiopterin (BH4 to BH4ox (BH2)) and several other mechanisms. 4A. These ROS/RNS can also feedback to indirectly potentiate further increases in NADPH oxidase 4 and iNOS. 4B. Prolonged increases in H2O2 stimulate an increase in eNOS expression that ultimately becomes uncoupled under the concurrent environment of increased ROS/RNS. 4C. The increased levels of uncoupled eNOS decrease its NO production and generate increased O2− levels that again react with H+ and NO to form H2O2, ONOO−, hydroxyl radical (OH−), nitrogen dioxide (NO2−) and several other ROS/RNS species. 5. These uncoupled eNOS-generated ROS/RNS feedback to uncouple more eNOS; 6A, and feedforward (in conjunction with decreased NO) to decrease the expression and activity of soluble guanylate cyclase (sGC), resulting in a decreased effect of the sGC product, cGMP, to promote vascular tissue vasodilation and health. 6B. These uncoupled eNOS-generated ROS/RNS products, along with those formed via increased NADPH oxidase 4 and iNOS feedforward to also directly cause damage to the vasculature (endothelium and smooth muscle cells) as listed in 6C. 7. Inflammatory cytokines and growth factors from infiltrating immunocytes and proliferating vascular smooth muscle cells further stimulate NADPH oxidase 4 and iNOS and these enzymes also feedback to stimulate these same actions. The metabolic syndrome milieu initiates and maintains the vascular production of ROS/RNS via multiple internal positive feedback and feedforward lops that ultimately lead to vascular damage and CVD. Prolonged increases in H2O2 production from NADPH oxidase 4, iNOS, and uncoupled eNOS stimulate the increased expression of GTPCH 1, the rate-limiting enzyme in BH4 synthesis, and BH4 is itself an end product, negative feedback inhibitor of GTPCH 1. As BH4 becomes oxidized by the prevailing ROS/RNS stress, less of it is available to inhibit GTPCH 1. Increased levels of GTPCH 1 are a biomarker of tissue pro-oxidative/nitrosative and proinflammatory state. NO2−—nitrogen dioxide; OH−—hydroxyl radical; PDGF—platelet-derived growth factor; EGF—epidermal growth factor; TGFβ—transforming growth factor beta; SNS—sympathetic nervous system; VSMC—vascular smooth muscle cell; BH4—tetrahydrobiopterin; BH4 ox—oxidized tetrahydrobiopterin;  —increases;
—increases;  —decreases;
—decreases;  —stimulates;
—stimulates;  —inhibits;
—inhibits; 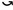 ,
, 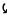 ,
,  ,
, 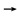 ,
,  ,
, 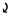 ,
,  ,
,  ,
,  ,
,  leads to;
leads to;  ,
,  collectively leads to these collective events. Enzyme levels and cardiometabolic parameters measured in the study are highlighted in yellow.
collectively leads to these collective events. Enzyme levels and cardiometabolic parameters measured in the study are highlighted in yellow.