Fig. 1.
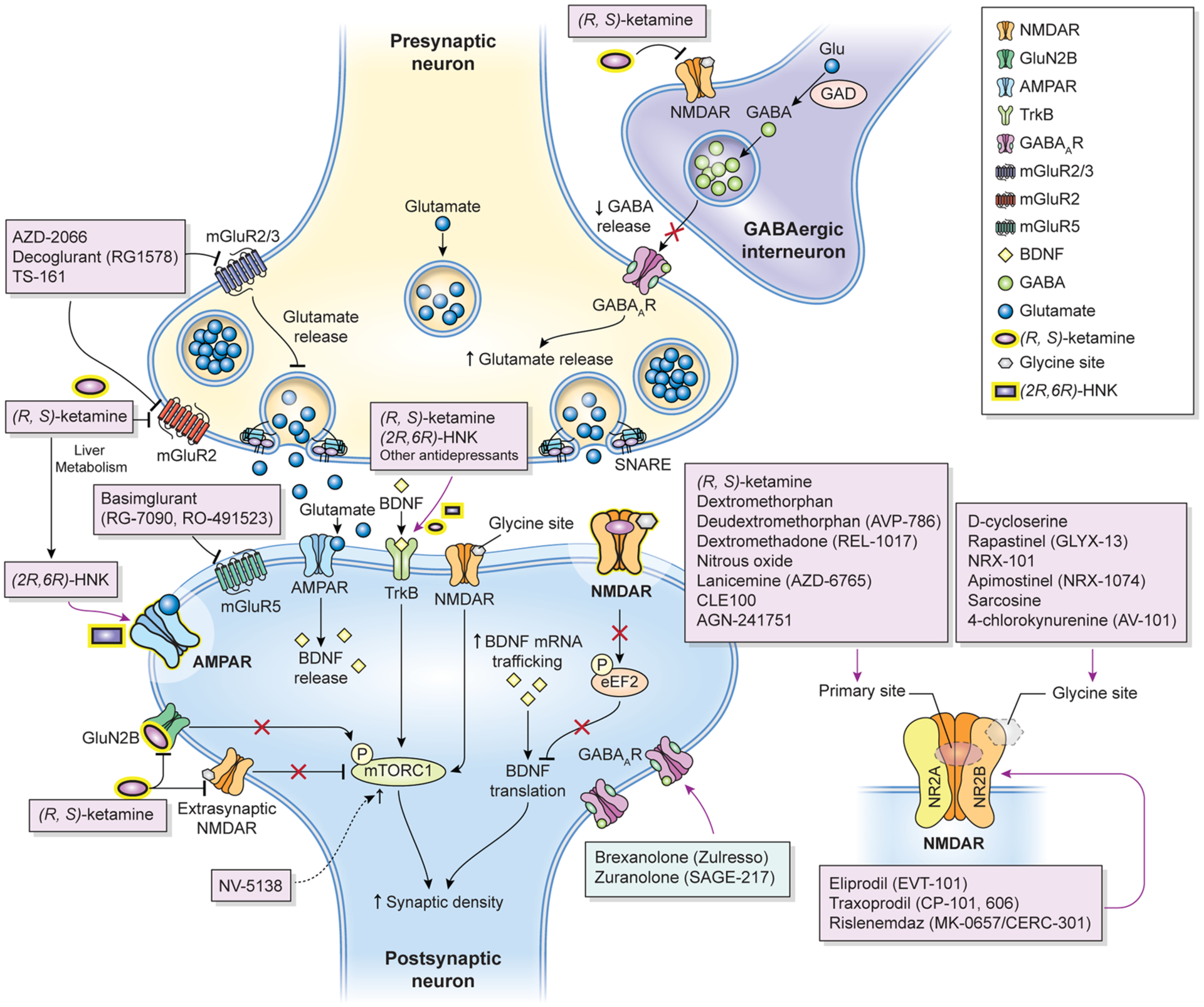
Proposed mechanisms of action of glutamatergic modulators and other putative rapid-acting antidepressants. Disinhibition hypothesis: Racemic (R,S)-ketamine and esketamine selectively block N-methyl-D-aspartate receptors (NMDARs). These are expressed on γ-aminobutyric acid (GABA)-ergic inhibitory interneurons, and their blockade decreases interneuron activity that, in turn, leads to disinhibition of pyramidal neurons and enhanced glutamatergic firing. Glutamate then binds to and activates post-synaptic α-amino-3- hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). Inhibition of spontaneous NMDAR-mediated transmission: Alternatively, (R,S)-ketamine may induce rapid brain-derived neurotrophic factor (BDNF) translation in the hippocampus, reduce phosphorylation, and activate eukaryotic elongation factor 2 (eEF2). Ketamine may also preferentially bind to NMDARs and affect neuronal NMDAR-mediating spontaneous excitatory transmission, which at rest keeps eEF2 phosphorylated and inhibits BDNF synaptic translation. De-suppression of BDNF translation then contributes to changes in synaptic plasticity that mediate (R,S)-ketamine’s antidepressant effects. AMPAR activation is also necessary for these effects. Inhibition of extra-synaptic NMDARs: Ketamine selectively blocks extra-synaptic GluN2B-containing NMDARs, which are tonically activated by low levels of ambient glutamate regulated by the excitatory amino acid transporter 2 (EAAT2) located on astrocytes. Inhibition of the extra-synaptic GluN2B-NMDARs de-suppresses mammalian target of rapamycin complex 1 (mTORC1) function, which in turn induces protein synthesis. Blockade of spontaneous NMDAR activation inhibits eEF2 kinase (eEF2K) activity, thus preventing phosphorylation of its eEF2 substrate. This effect subsequently enhances BDNF translation and, ultimately, protein synthesis. The role of ketamine metabolites: (2R,6R)-hydroxynorketamine (HNK) exerts NMDAR inhibition-independent antidepressant actions by activating AMPAR-mediated synaptic potentiation. Other glutamatergic modulators: Metabotropic glutamate receptor (mGluR) 2/3 antagonists are thought to enhance synaptic glutamate levels, thereby boosting AMPAR transmission and firing rates and extracellular monoamine levels. GLYX-13, which has partial agonist properties at NMDARs, is hypothesized to activate mTORC and subsequently induce protein synthesis. GLYX-13 requires AMPA and activity-dependent BDNF release but, unlike (R,S)-ketamine, it does not produce glutamate bursts. AMPAR activation enhances BDNF release, activates the tropomyosin receptor kinase B (TrkB) receptor, and subsequently promotes protein synthesis by activating the mTORC complex. GABAAR GABA type A receptor, GAD glutamate decarboxylase, Glu glutamate, SNARE soluble N-ethylmaleimide-sensitive factor attachment protein receptor Adapted with permission from Kadriu et al. [5]
