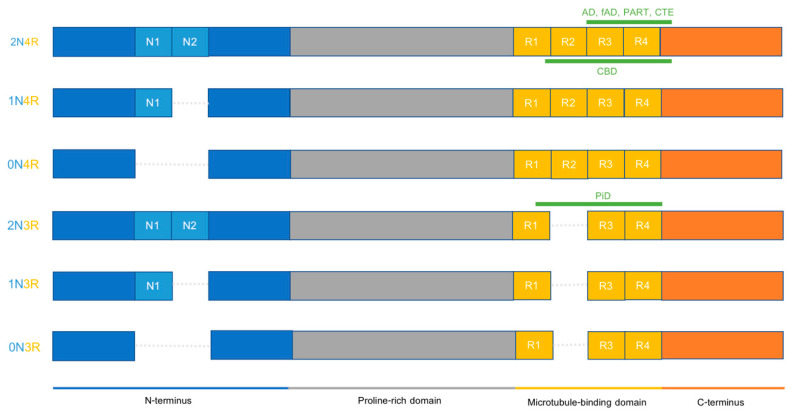
Figure 1.
Tau isoforms in the adult human CNS and their relation to disease-specific tau fibrils. Tau filaments from distinct tauopathies exhibit different structural features. AD, fAD, and PART have identical cores spanning amino acids 306 to 378 that can incorporate all six tau isoforms (tau core was only indicated on the 2N4R isoform but applies to all six isoforms). These filament cores include repeats 3 and 4 and a portion of the C-terminus. The CTE core has some similarity to the AD core in terms of incorporated regions (R3 and R4) but is structurally distinct and is located between amino acids 305 to 379. CBD tau fibrils have a core that spans R1 until 12 residues after R4 and can only include 4R isoforms (tau core was only indicated on the 2N4R isoform but applies to all three 4R isoforms). CTE cores include amino acids 274 to 380. PiD tau fibrils have a core that spans R1 to R4 of 3R tau isoforms, between amino acids 254 and 378 (tau core was only indicated on the 2N3R isoform but applies to all three 3R isoforms). This core excludes R2, explaining why this is a 3R tauopathy.