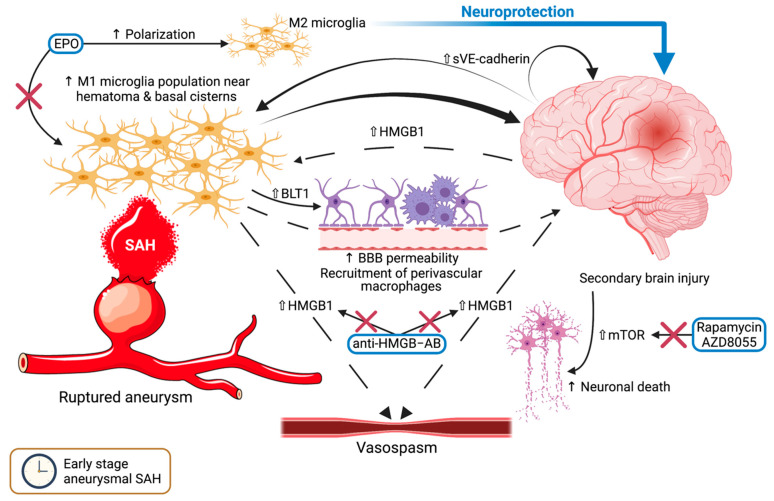
Figure 1.
The mechanisms and pathways involving microglia in the early stage of subarachnoid hemorrhage. Following aneurysmal rupture, the blood in the subarachnoid and basal cisterns leads to an increased population of pro-inflammatory M1 microglia, which in turn will lead to secondary brain injury, heightened blood-brain barrier permeability, and recruitment of perivascular macrophages. Upregulated HMGB1 within the brain sustains this process and possibly leads to the occurrence of vasospasm, while anti-HMGB antibodies interrupt this cycle. VE-Cadherin maintains secondary brain injury and M1 microglia activation. Erythropoietin sways the polarization toward the M2 phenotype, resulting in a neuroprotective effect. Moreover, an upsurge of mTOR results in elevated neuronal death. Rapamycin and AZD8055 both inhibit the effects of mTOR. A transparent upward-pointing arrow denotes upregulation, whereas a thin upward-pointing arrow signifies an elevation of the mentioned process. Red crosses mark an inhibitory effect. Abbreviations (in alphabetical order): BBB, blood-brain barrier; BLT1, leukotriene B4 receptor 1; EPO, erythropoietin; HMGB1, high-mobility group box 1; HMGB1–AB, HMGB1-antibodies; mTOR, mammalian target of rapamycin; SAH, subarachnoid hemorrhage; sVE-Cadherin, soluble vascular endothelial cadherin.