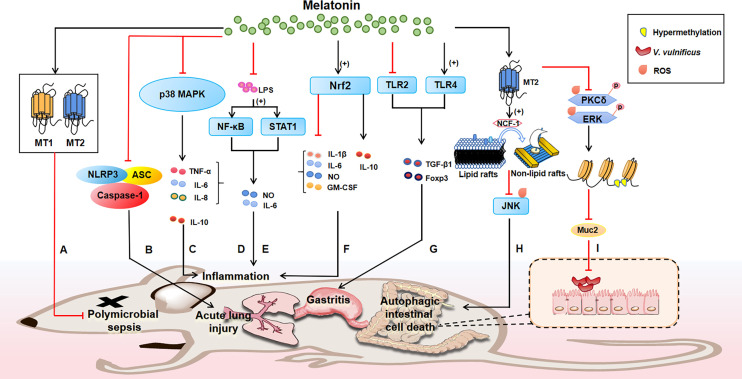
Figure 2.
The bacteriostatic mechanisms of melatonin in animals. Melatonin receptors (e.g., MT1 and MT2) play a great role in mediating the functions of melatonin. Melatonin reduces polymicrobial sepsis through MT1 and MT2, thus improving the survival rate of mice (A); notably, the mechanisms whereby melatonin exerts bacteriostatic action in vivo are tightly associated with the immune responses, for instance, melatonin inhibits NLRP3 inflammasome to alleviate acute lung injury (B); melatonin reduces pro-inflammatory cytokines, increases anti-inflammatory cytokines and improves survival through p38MAPK signaling pathway (C); melatonin blocks LPS-induced activation of NF-κB (D) and STAT1 (E), thus inhibiting the production of inflammatory factors to modulate inflammation. Likewise, suppression of inflammation also occurs when melatonin activates Nrf2, evidenced by reduced pro-inflammatory mediators (e.g., IL-1β, IL-6, NO and GM-CSF) and increased anti-inflammatory cytokine (e.g., IL-10) (F); melatonin treatment can target TLR to alleviate H. pylori-induced gastritis by promoting TLR4 and inhibiting TLR2 to regulate TGF-β1 and Foxp3 expression (G); of note, melatonin could be a therapeutic alternative agent to fight bacterial infections due to its antioxidant function. For example, melatonin signaling via MT2 promotes NCF-1 recruitment from lipid rafts to non-lipid rafts to block the ROS-mediated JNK pathway, preventing autophagic intestinal cell death (H); moreover, MT2 signaling inhibits the ROS-mediated phosphorylation of PKCδ and ERK to reduce region-specific hypermethylation in the Muc2 promoter, combating V. vulnificus infection (I). Note: plus in parentheses represents increase; and the red line represents inhibition; the black arrow indicates activation.