Scheme 1. Synthesis of Compounds 11 (A) and 19 (B).
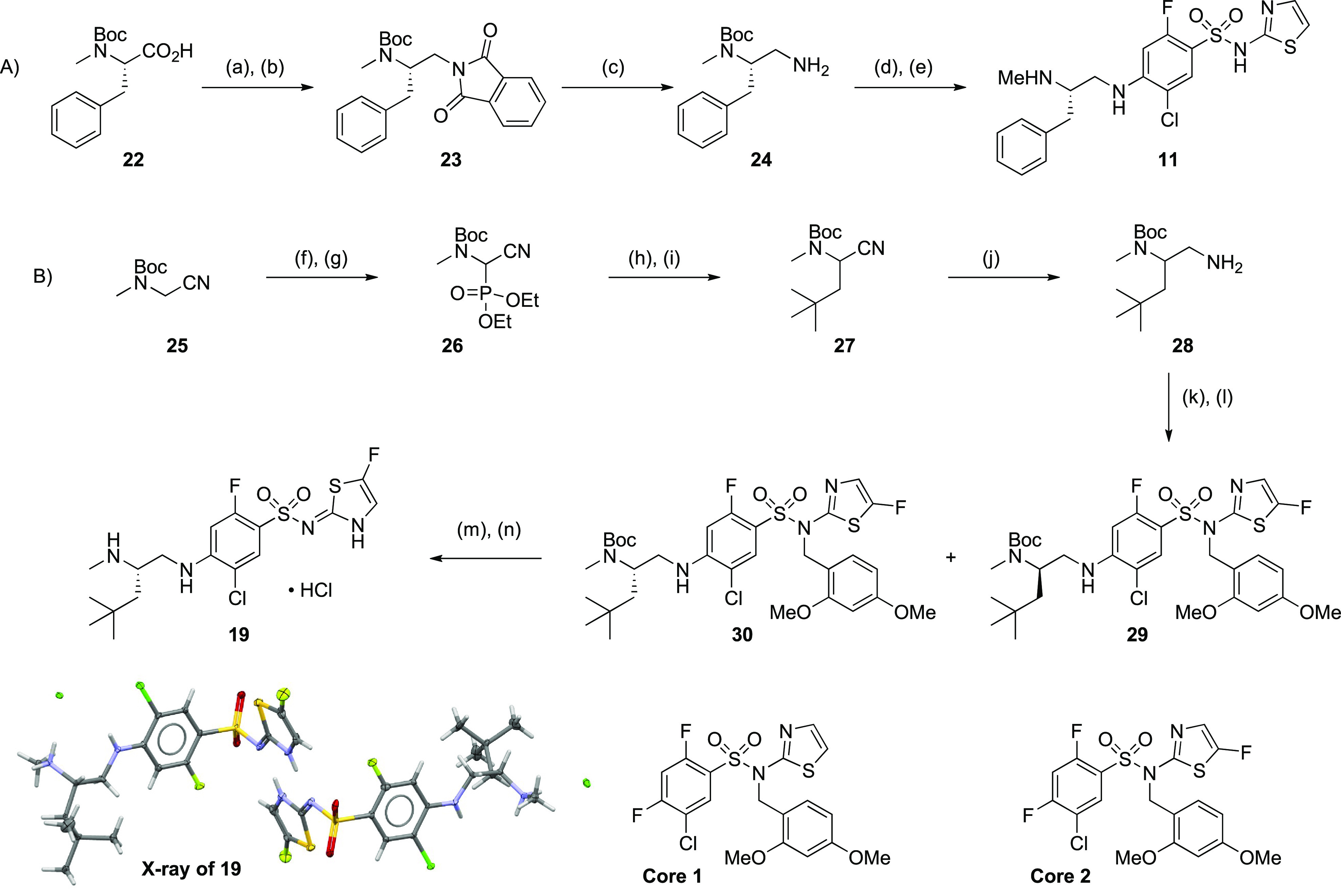
Reagents and reaction conditions: (A) (a) BH3·THF, THF, 0–25 °C, 0.5 h, 97%; (b) phthalimide, DTAD, PS–PPh3, THF, 25 °C, 0.5 h, 78%; (c) hydrazine hydrate, MeOH, reflux, 2 h, 97%; (d) core 1, Hunig’s base, NMP, 100 °C, microwave, 20 min, 77%; (e) TFA, DCM, 25 °C, 3 h, 79%. (B) (f) N-bromosuccinimide, CCl4, 80 °C, 2 h 96%; (g) triethylphosphite, THF, 75 °C, 16 h, 98%; (h) pivaldehyde, tetramethylguanidine, THF, −78 to 25 °C, 9 h, 57%; (i) NaBH4, MeOH, 25 °C, 2 h, 64%; (j) Raney nickel, H2, EtOH, 25 °C, 2 h, 63%; (k) core 2, TEA, DMF, 25 °C, 16 h, 83%; (l) SFC separation, Chiralpak AD-3 150 mm × 4.6 mm I.D.; mobile phase, 2-propanol (0.05% DEA) in CO2 from 5% to 40%; flow rate, 2.4 mL/min; wavelength, 220 nM; enantiomer 1 = 30 (Rt = 5.0 min), eantiomer 2 = 29 (Rt = 5.4 min); (m) TFA, DCM, 25 °C, 1 h; (n) reverse phase HPLC (acetonitrile/0.05% HCl in water), 61% over 2 steps.
