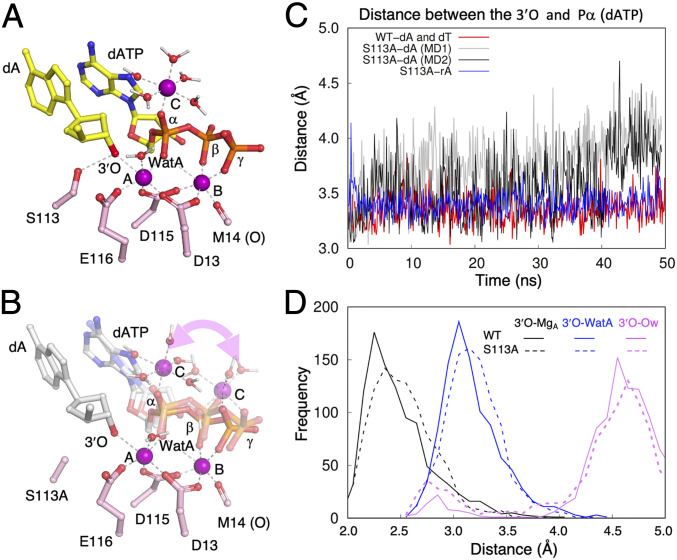
Fig. 5.
MD simulation of the WT and S113A active sites. (A) The C-site Mg2+ is stably bound to the α-phosphate of dATP in the WT active site. Three Mg2+ ions (A, B, and C) are shown as purple spheres. The primer-end dA, dATP, catalytic residues, and inner-shell water molecules of Mg2+ ions are shown as sticks, and Mg2+ coordinations are shown as dashed lines. (B) In multiple independent MD simulations of the S113A mutant, the C-site Mg2+ binds either the α- or β-γ phosphates of dATP. The alternative binding mode is indicated by the curved double arrowhead. The incoming dATP also wobbles during simulations. Two representative intermediate states are shown. (C) During dynamics simulation, the distance between the primer O3′ and Pα of dATP is stable in the WT Pol η active site (red trace) but is varied and longer in the S113A active site. Two traces of the S113A simulation are shown in gray and black. In the S113A-rA simulation, the two reactants (O3′ and Pα of dATP) are as stable (blue trace) as with WT Pol η. (D) Comparison of distances between the O3′ and Mg2+A, WatA, and surrounding water molecules in the WT (solid lines) and S113A mutant Pol η (dashed lines).