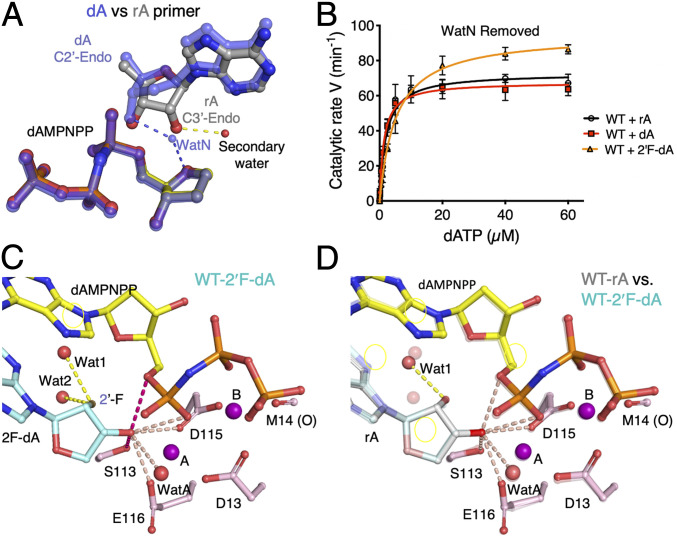
Fig. 6.
Deprotonation route is flexible. (A) Structural superposition of the dAMPNPP-bound WT Pol η with dA- (semitransparent blue sticks) and rA- (gray and yellow sticks) primers. The transient water associated with the 3′-OH of dA is shown. The secondary water of the rA structure is shown as a red sphere. Hydrogen bonds between the terminal primer sugar hydroxyl groups and the water molecules are shown as dashes. (B) Steady-state kinetic profiles of WT Pol η extending primers terminating in dA, rA, or 2′F-dA. Kinetic parameters for each profile are listed in Table 1. Error bars represent standard deviation of triplicate measurements. (C) Possible 3′-OH deprotonation routes. The structure of WT Pol η bound to the 2′F-dA–terminated primer is shown. The six atoms within 3.1 Å of the 3′-OH of 2′F-dA are marked by pink dashed lines. Two (the hydroxyl of S113 and O5′ of dAMPNPP) are highlighted in hot pink because these distances are 0.1 to 0.15 Å shorter in the 2′F-dA complex than those in the rA complex. (D) The structure of the rA-primer is superimposed on 2′F-dA (semitransparent). The two are nearly identical. Two water molecules (Wat1 and Wat2) are within hydrogen-bond distance of the 2′-F. Only Wat1 is bound to the 2′-OH of rA.