Fig. 6. Tests of CAR T cell function in recipient mice.
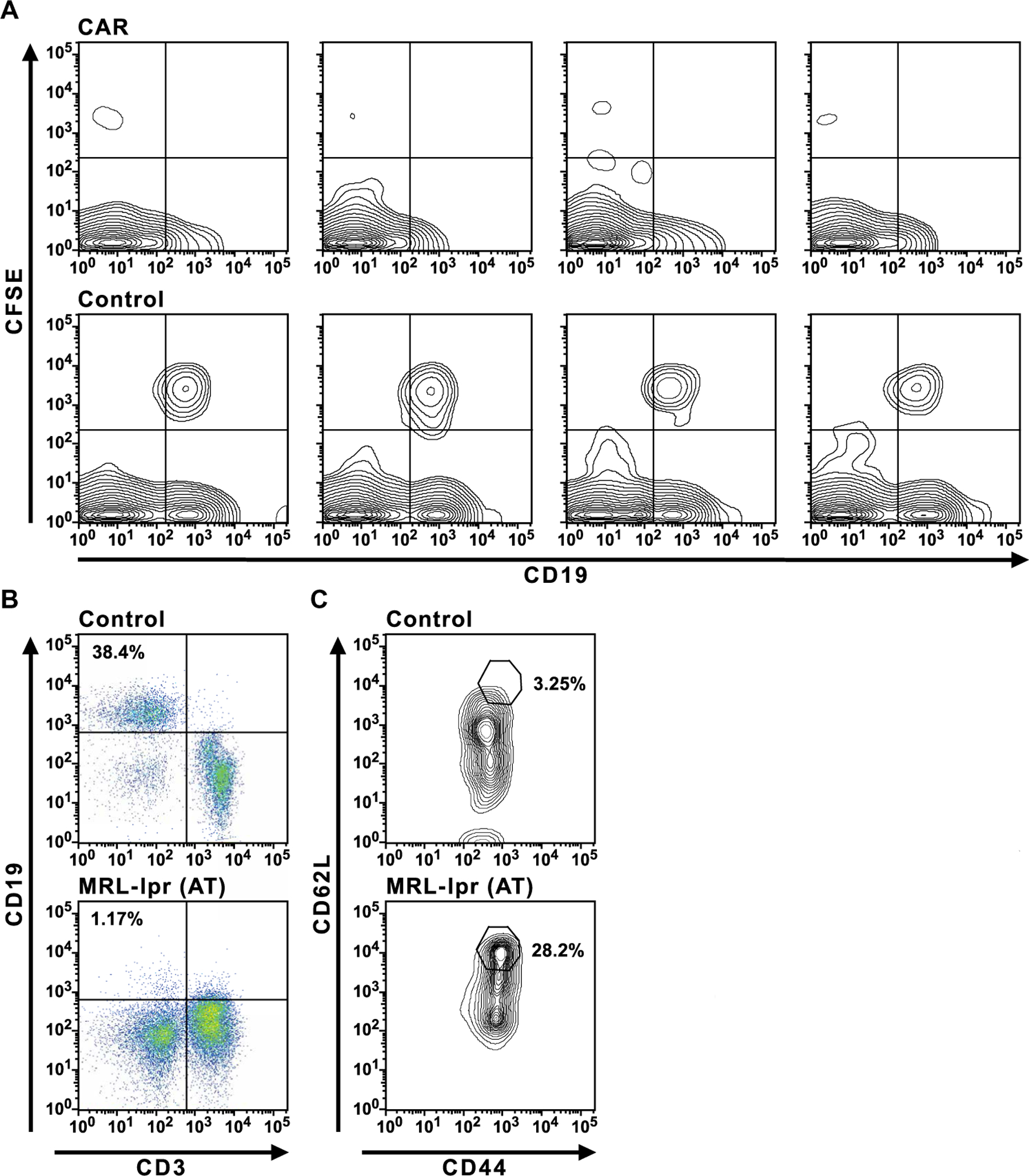
(A) Injections of CFSE-labeled B cells into control or CAR T cell–treated MRL-lpr mice were performed 4 months after CAR T cell administration. Six days after injection into control or CAR T cell–treated mice, peripheral blood was assayed for labeled B cells by flow cytometry. (B and C) Adoptive transfer (AT) of purified splenic CD8+ T cells from an MRL-lpr mouse that was treated 7 months earlier with CD19-targeted CAR T cells into a second group of previously untreated, 2-month-old MRL-lpr mice. The secondary MRL-lpr recipients (MRL-lpr AT) and control MRL-lpr mice were assayed for CD19+ B cells (B) in peripheral blood 4 months after transfer and CD44hiCD62Lhi CD8+ T cells (C). The data in (B) and (C) are representative of four MRL-lpr AT mice observed in two experiments.
