Significance
The substrate translocation through the proteasomal AAA+ motor is central to the process of protein degradation. Emerging structural studies challenge one to advance a detailed knowledge of the energetics of the translocation process. Here, we generate the relevant free energy landscape using cryogenic electron microscopy structures of the substrate-bound 26S proteasome by using a combination of coarse-grained modeling, simplified atomistic binding free energy calculations, and ATP hydrolysis energies. This landscape successfully reproduces the unidirectionality of the biological process and reveals that the unidirectional motion can be significantly enhanced/tuned by electrostatic effect. Our mutagenesis analysis identifies key residues of pore loop 1 and estimates their contribution to the translocation process. This work provides a guidance for investigating ATP-driven translocation processes of proteasomes.
Keywords: proteasome, ATPase, molecular motor, protein degradation, AAA+
Abstract
This work explored the molecular origin of substrate translocation by the AAA+ motor of the 26S proteasome. This exploration was performed by combining different simulation approaches including calculations of binding free energies, coarse-grained simulations, and considerations of the ATP hydrolysis energy. The simulations were used to construct the free energy landscape for the translocation process. This included the evaluation of the conformational barriers in different translocation steps. Our simulation reveals that the substrate translocation by the AAA+ motor is guided in part by electrostatic interactions. We also validated the experimental observation that bulkier residues in pore loop 1 are responsible for substrate translocation. However, our calculation also reveals that the lysine residues prior to the bulkier residues (conserved along pore loop 1) are also important for the translocation process. We believe that this computational study can help in guiding the ongoing research of the proteasome.
The 26S proteasome (Fig. 1) is the major protease in eukaryotic cells. This system is not only responsible for protein degradation in both the cytosol and the nucleus but also controls ample cellular processes including DNA replication, transcription, stress response, cell cycle, and signal transduction (1). The ubiquitin–proteasome system is the central pathway in controlling cytosolic protein quality, where the bulk of the protein degradation (up to 90%) (2) in the nucleus of eukaryotic cells is achieved. Misfolded or short-lived as well as potentially toxic proteins are identified through covalent attachment of the polyubiquitin chains to the lysine residue. Subsequently, they are pulled where the higher-order structures disrupted and then the unfolded polypeptides are translocated to the proteolytic center (3) for degradation. The role of ubiquitin in the proteolytic pathways and the importance of proteolytic degradation inside cells was acknowledged in the award of the 2004 Nobel Prize in Chemistry to Aaron Ciechanover, Avram Hershko, and Irwin Rose (4, 5).
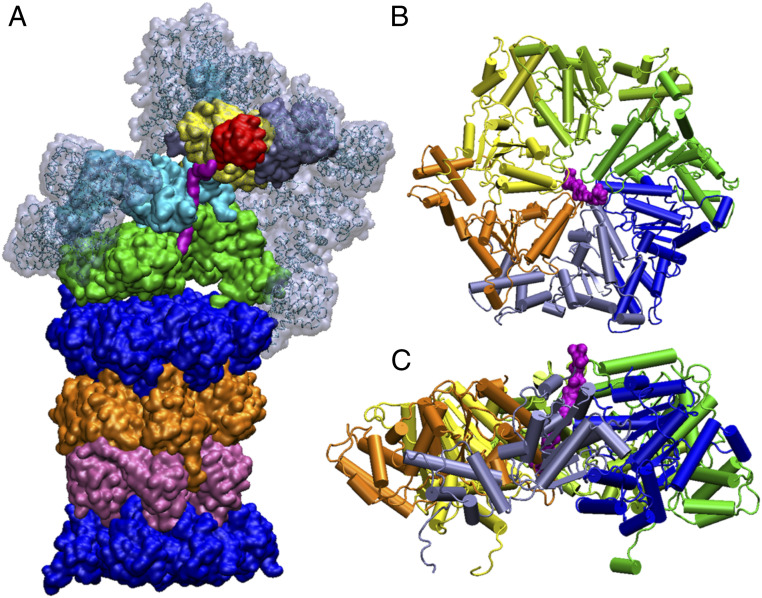
Fig. 1.
Illustration of the structure of different parts of the 26S proteasome. (A) Transparent gray, ice blue, red, yellow, cyan, magenta, green, top blue, orange, mauve, and bottom blue denote, respectively, the non-ATPase regulatory subunits, the ubiquitin, the RPN11, the OB ring of the AAA+ motor, the substrate, the ATP-fueled AAA+ motor, and the 7, β5, β5, 7 system. The upper section (transparent gray, ice blue, red, yellow, cyan, magenta, and green) forms the 19S RP. The bottom section (blue, orange, mauve, and bottom blue) forms the 20S core particle. Two coiled coils of the AAA+ motor are omitted here for a better view of the ubiquitin-bound substrate. (B) Top view of the AAA+ motor. (C) Side view of the AAA+ motor.
As a multicomponent and compartmental protease of the AAA+ ATPase family, the 26S proteasome has a complicated architecture which assembles multiple layers of regulatory and functionally diverse adaptor proteins around the AAA+ core (Fig. 1). The main proteasome species in eukaryotes, the 26S proteasome, is composed of two main components: a 20S catalytic core particle and a 19S regulatory complex, overall a 2.5-MDa protein complex. The 20S core particle is capped by 19S regulatory particles (RPs) at both ends and has a molecular weight of ∼700 kDa (6). The 20S core particle has a cylinder or barrel shape, composed of four stacked rings, each of which is made of seven heteromeric subunits. These heteromeric subunits contain one or two different α and β subunits, where the β subunit consists of 211 residues and the α subunit consists of 233 amino acids. Crystal structures of several mammalian 20S proteasome demonstrated that the cylindrical 20S particle contains two identical halves in an α7β7β7α7 arrangement (1). Each of the identical halves is made of 14 unique proteins which have an individual molecular weight of ∼20 30 kDa. The 20S core particle is similar to a hollow barrel with a diameter and length of 11 and 15 nm, respectively. The volume of the central chamber inside the 20S core particle is ∼84 nm3 and formed of two inner β rings, which is often interestingly compared with a folded protein with molecular weight of ∼70 kDa (1). Several crystal structures (with and without inhibitors) have illustrated that the system has a huge potential to allow larger polypeptides to enter the antechamber, which has a volume of ∼60 nm3 and is formed by two innermost β rings and two outermost α rings arranged in α7β7 order (1, 7). The 19S RP has two main structural sections: lid and base. The lid contains nine non-ATP-dependent subunits (Rpn3, Rpn5, Rpn6, Rpn7, Rpn8, Rpn9, Rpn11, Rpn12, and Rpn15). The base is composed of 10 subunits where six homologous AAA–ATPase (adenosine triphosphatases, called Rpt 1 to 6, where Rpt stands for RP ATPase) subunits form a ring (8–11). Each of these Rpt units consists of an N-terminal alpha helix and an OB (oligonucleotide/oligosaccharide binding) domain, which stays above the AAA+ ring domain. The remaining four subunits in the base are non-ATP-dependent subunits called Rpn1, Rpn2, Rpn10, and Rpn13 (RP non-ATPase).
While the Rpn units serve a variety of functional responsibilities regarding ubiquitylation and deubiquitylation, the heterohexameric AAA+ ATPase domain plays a crucial role in unfolding and translocating the client substrate protein (12). The AAA+ ATPases (associated with diverse cellular activities) is a superfamily of proteins which has a conserved ATPase domain and acts like a machine by converting the energy of ATP hydrolysis into mechanical force that leads to a large-scale conformational rearrangement (13). This multiskilled engine-like module serves a range of different biological functions, including DNA replication where single strands of nucleic acid are unfolded by an AAA+ helicase, viral genome packaging where DNA or RNA is pumped into the protein capsids, and protein quality control where misfolded protein target is identified, unfolded, and translocated for degradation through the AAA+ enzyme (14).
Although the AAA+ domains are highly conserved across species, its superfamily is classified into seven different classes exclusively based on their unique ability to insert and process different biological entities inside/through themselves (15). The nature of the remodeling of nucleic acids by the AAA+ motor has received intense research focus and evolved in crystallographic studies of DNA/RNA–bound AAA+ motors (16). However, the nature of action of the protein substrate-bound AAA+ motor has been elusive for a long time. Thus, the mechanism of proteasomal ATPases in eukaryotes and archaea remained poorly understood until recently when cryogenic electron microscopy (cryo-EM) technology breakthroughs revealed a plethora of detailed structural information in different parts of this protein (15). These high-resolution structures reveal that the substrate is threaded into the AAA+ motor through a narrow-gated channel, and thus translocation requires ATP-dependent unfolding of the substrate. It is interesting to note that this mechanism of unfolding and translocation is conserved across the AAA+ superfamily; however, it is exclusively regulated in a fine-tuned way to assist the host protein to perform its particular biological function.
The substrate-bound AAA+ protein translocases belong to the classical clade (among the seven different clades) according to all the cryo-EM structures released so far (15). Despite the highly conserved nature of the ATPase module, these classical AAA+ proteins can again be classified into four types based on their integration and hence organization with other unrelated domains required for specific regulatory activities across different species giving rise to their functional diversity (15). Type I ATPase (e.g., Vps4, Spastin) contains a single ATPase domain (17) and Type II ATPase (p97, VAT, PEX1-PEX6, NSF, Hsp104) contains two ATPase domains (18). While the primary molecular activity of Type I and II ATPases involves either disassembly or dislocation or extraction or disaggregation of biopolymers, the third type of ATPases is involved in degradation of proteins. While the AAA+ proteins, which degrades mitochondrial proteins, are categorized as AAA+ proteases, degradation of cytosolic and archaeal proteins is accomplished by AAA+ proteins, categorized as proteasomal AAA+ (19). Now, the cytosolic protein quality is governed by the ubiquitin–proteasome system, a multicomponent system that assemble multiple layers of adaptor and regulatory proteins which directly and indirectly influences the function of the proteasomal AAA+ motor. The 26S proteasome which is presented in the cytosol of the eukaryotes degrades certain proteins which are targeted and brought to them for degradation from different membranes by other types of AAA+ proteins.
It is reported multiple times in the literature (15) that substrate translocation by the AAA+ motor is characterized by several important aspects such as conserved residues along pore loops 1 and 2 of the AAA+ motor across several species, a hand-over-hand mechanism, sequential ATP hydrolysis, structure and charge of the substrate, and intersubunit communication of the Rpt units in the AAA+ motor. Since the structure of the substrate-bound human 26S proteasome was determined very recently (20, 21), many unanswered questions can be explored by computational models. Thus, in the context of the above structural and experimental knowledge we attempt here to simulate the substrate translocation in the 26S proteasome and find answers for key unanswered questions regarding the proteasome.
Despite immense structural advances in recent times, it is still uncertain how protein conformational changes in the AAA+ motor, which are triggered by ATP hydrolysis, lead to the translocation of the substrate. Thus, we focused here on the translocation of the substrate, trying to explore the structure/energy basis for this process. Our study produced the expected unidirectional motion. This was done while calculating the barriers involved in each step of the translocation process. We also explored the effect of mutations on the translocation energy profiles. Our mutagenesis analysis has been found to be consistent with the corresponding experimental facts.
Results and Discussion
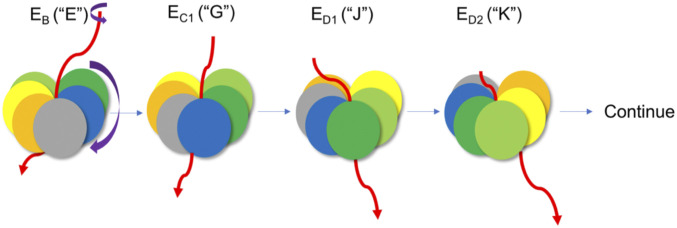
This paper focuses solely on the role of the AAA+ machinery (the motor module of the system) of the human proteasome in the functional pathway. A cartoon representation of substrate translocation through the 26S proteasome is provided in Fig. 2, which qualitatively illustrates how the Rpt units move in a clockwise direction while the substrate translocases in a counterclockwise direction. This is also illustrated with explicit structures in Fig. 3. The nucleotide states of the color-coded Rpt units in the AAA+ motor are described in Fig. 4. The clockwise movement of the Rpt units and the resulting stepwise translocation of the substrate driven by sequential ATP hydrolysis cycle that proceeds counterclockwise around the hexameric ring have been reported multiple times (8, 20, 21) and are nicely illustrated in ref. 15.
Fig. 2.
Cartoon representation of the substrate translocation through the 26S proteasome AAA+ motor. Deep green, blue, gray, orange, yellow, and light green represent, respectively, the Rpt3, Rpt4, Rpt5, Rpt1, Rpt2, and Rpt6. The red line represents substrate. Each structure from the left-hand side represents state EB, EC1, ED1, and ED2, respectively. The color schemes of the Rpt units are consistent throughout the entire paper. The structures of EB, EC1, ED1, and ED2 are designated as structures “E,” “G,” “J,” and “K” throughout the paper. The violet arrow demonstrates the clockwise motion of Rpt units as the substrate translocates in a somewhat “counterclockwise” direction.
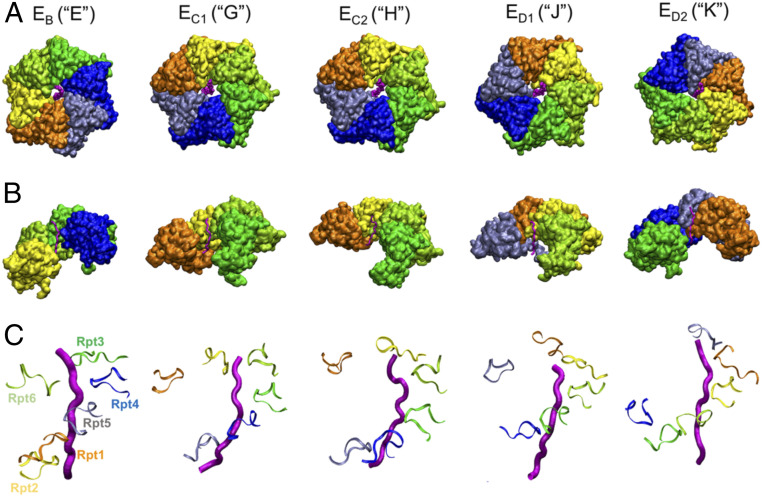
Fig. 3.
(A) Top view of the 26S proteasome AAA+ motor. (B) The side view of the 26S proteasome AAA+ motor (in each structure the front two Rpt units are omitted for better view of the substrate). (C) Pore loop 1 showing nine residues from each Rpt unit around the substrate. Deep green, blue, gray, orange, yellow, and light green represent Rpt3, Rpt4, Rpt5, Rpt1, Rpt2, and Rpt6, respectively. Pink tube represents the substrate. The structures, starting from the left-hand side, represent states EB, EC1, EC2, ED1, and ED2, respectively. The color schemes of the Rpt units are kept the same throughout the entire paper. The structures of EB, EC1, EC2, ED1, and ED2 are designated as structures “E,” “G,” “H,” “J,” and “K” throughout the paper.
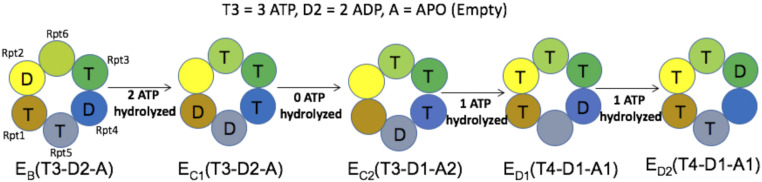
Fig. 4.
The nucleotide states of the 26S Proteasome AAA+ motor. The structures of EB, EC1, EC2, ED1, and ED2 are designated as structures “E,” “G,” “H,” “J,” and “K” throughout the paper. Color schemes of Rpt units are the same throughout the paper.
Our study started with five cryo-EM structures of the substrate-bound AAA+ motor of the 26S human proteasome reported in ref. 20, while truncating Rpt1, Rpt2, Rpt3, Rpt4, Rpt5, Rpt6, and the substrate (details are provided in Materials and Methods). The resulting system was used in studying the structure and function of the AAA+ motor system and its interaction with the substrate and in understanding the translocation process.
The Free Energy Landscape.
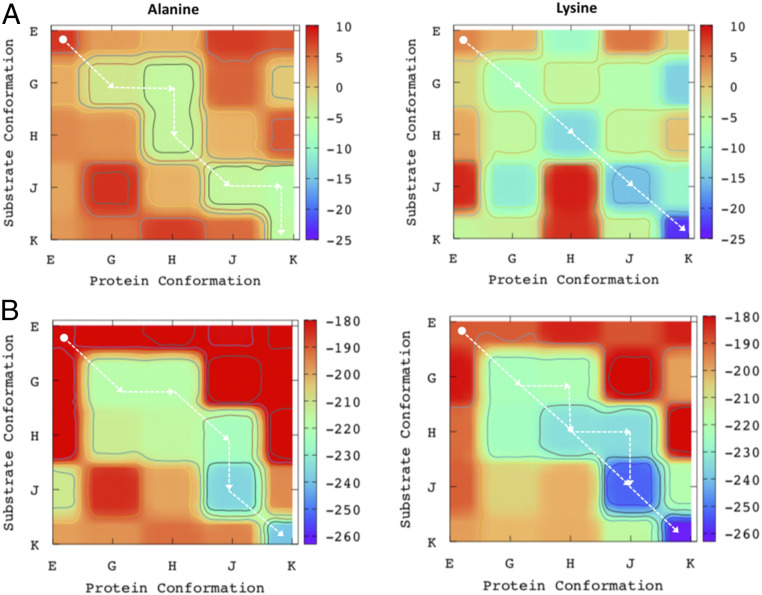
Looking at the structure depicted in Fig. 2, it is reasonable to assume that the substrate moves thorough the pore loop in a “directional translocation” manner. However, in order to substantiate such an assumption, it is essential to obtain a directional translocation by evaluating the relevant structure/energy relationship. As explained in our previous report (22), in order to understand the mechanism/action of a translocase it is very helpful to start with a hypothetical model that examines the conditions for a translocation process. The corresponding analysis of the hypothetical translocase and its free energy landscape is presented in SI Appendix, Figs. S1 and S2 and discussed briefly therein. Based on similar considerations, we constructed a hierarchical free energy surface considering different energy contributions. To construct the relevant free energy surface, we started with the five structures of the AAA+ (20). The change in the substrate conformation is defined as the y axis and the change in the protein conformation is defined as the x axis of Fig. 5. We first evaluated the free energy landscape of the binding of the substrate to the AAA+ motor (Fig. 5A). The substrate in Fig. 5 A, Left is entirely hydrophobic, consisting of 13 alanine residues. To investigate the specificity of the substrate in the translocation process we also simulated a substrate containing 12 alanine residues and 1 lysine substrate. Fig. 5 A, Right shows the surface for the substrate where the lone lysine residue is present at the front end penetrating through the pore loop. The binding energy of the substrate was evaluated using the semimacroscopic version of the protein–dipole Langevin–dipole model with the linear response approximation (LRA) treatment with a scaled nonelectrostatic term (PDLD/S-LRA/) (23). A one-dimensional free energy landscape is also provided in SI Appendix, Fig. S3.
Fig. 5.
The free energy landscape at different hierarchical energy components for the “Alanine” (Left) and “Lysine” (Right) substrate systems. (A) The free energy landscape contribution due to the substrate binding (evaluated by the PDLD/S-LRA/ method). (B) The sum of the free energy contributions to the landscape due to the substrate binding (evaluated by the PDLD/S-LRA/ method), electrostatic energy of the entire AAA+ motor (evaluated by the CG method), and the contributions of the ATP energies. The “Alanine” column represents the results for the substrate containing 12 alanine residues and the “Lysine” column represents the results for the substrate containing 12 alanine residues and 1 lysine residue. The AAA+ motor structural changes are defined as the “Protein Conformation” on the x axis and the translocation of the substrate is defined as the “Substrate Conformation” on the y axis. Structures EB, EC1, EC2, ED1, and ED2 are designated as structures “E,” “G,” “H,” “J,” and “K” throughout the paper.
The calculated surface shown in Fig. 5 A and B, as well as in SI Appendix, Fig. S2, indicates that an energetically viable path opens up diagonally for the substrate penetration and produces the specific directionality of the system. The protein–substrate interaction is much stronger in subsequent stages as compared with the initial “E” state. Thus, this surface suggests a narrow channel along the diagonal direction (downhill energetic) which is accessed by the system. It is interesting to notice that although both “E” and “G” structures belong to the same nucleotide state (T3-D2-A as shown in Fig. 4), the substrate binding is much weaker when the opposite Rpt monomers (Rpt2 and Rpt4 in the “E” structure) are in the ADP states. Considering the protein–substrate binding surface, we can see that the access of the off-diagonal points at state EC2 (structure H) and ED2 (structure K) charts a unique route for the substrate penetration through the pore loop, indicating the fact that transformation from E to G and H to J follows the diagonal, whereas in the case of transformation from G to H and J to K the off-diagonal protein conformations have relatively more wiggle room to host the substrate during the translocation. Comparing the surfaces of neutral and charged substrate, we notice that while in the case of charged substrate (Fig. 5A) the system will follow strictly the diagonal direction, in the case of the neutral substrate the system will access a specific route involving couple of off-diagonal states. It is interesting to notice that the overall directionality is more pronounced in the case of charged substrate. This result is consistent with the experimental observation (24) that substrates with neutral/positive charges exhibit accelerated rates of unfolding.
Note, however, that detailed experiments (25) with well-defined substrate show very moderate change in degradation upon change in sequence. This correlates with our simulation results which found that the initial barriers (“E” to “G” in Fig. 5 A and B) are similar for both “Alanine” and “Lysine” substrate. Nevertheless, the effect of charge on the degradation rate cannot be ignored, since both Sauer and coworkers (25) and Kudriaeva et al. (26) examined the effect of the charge on the substrate in nonhuman proteasomes and observed reasonably accelerated rate of degradation.
If the substrate specificity can be controlled by tuning its charges it may have potential applications in drug discovery. For example, it should promote targeting protein degradation, which is a rapidly emerging novel concept in cancer therapy. In this respect we note that Hilvert and coworkers (27) demonstrated that an artificial proteasome favors the uptake and hydrolysis of positively charged peptides and proteins, while excluding negatively charged competitors based on electrostatic interactions. These workers suggested that the protease/cage complex functions like an artificial proteasome in compartmentalization, enabling spatially and temporally controlled protein degradation. Here, the system relies on passive transport of the substrate along an electrostatic gradient to promote preferential uptake and hydrolysis of positively charged peptides and proteins and to discriminate against zwitterionic or negatively charged molecules.
The translocation of the substrate through the proteasomal AAA+ motor protein happens with a strong coupling between the substrate progression and the protein structural changes, which results in a substantial conformational change of the Rpt units during the translocation [as mentioned multiple times in the literature (15) and described as a rigid body motion of certain Rpt units while keeping others highly flexible]. With the current computing resources it is unlikely that one can accurately and meaningfully capture the overall free energy change of this large biological motor with a fully microscopic model and reach a reasonable convergence. Thus, coarse-grained (CG) models definitely represent an attractive opportunity for the treatment of such systems. It is, however, important to realize that without the inclusion of appropriate electrostatic terms (28, 29) it is highly unlikely to achieve a meaningful free energy landscape. Thus, we have used our reliable CG model which is focused on a consistent description of electrostatic energy of the system (30). Since our model has been effective in reproducing protein folding energies (31, 32) and the complicated action/mechanism of several biological processes [including F1/F0 ATPases (33) and helicases (22)] we believed that it should be effective in studying the mechanism of the proteasomal AAA+ motor protein and its underlying physics. Our CG modeling of the substrate-bound proteasomal AAA+ motor system appeared to reproduce a very reasonable unidirectional free energy surface (SI Appendix, Fig. S4, “Alanine_CG” and “Lysine_CG” column) for the substrate translocation without applying unrealistic forces or using phenomenological parameters. Thus, we started by adding the CG electrostatic energy of the entire system to our two-dimensional (2D) free energy landscape (SI Appendix, Fig. S4).
In order to obtain the overall free energy surface it is essential to also include the ATP internal energy (SI Appendix, Fig. S4). The surface obtained with this contribution is shown in Fig. 5B, where we have added the CG and ATP energies to the PDLD/S-LRA/ binding energies. Our consideration of the required number of ATP molecules for each transition is based on the nucleotide states (Fig. 4) from the structural revelation in ref. 20. The transfer from state “E” to “G” involves –8 2 kcal/mol (Fig. 4) since it accounts for a change from (ATP + water) to (ADP + Pi) in aqueous solution for two ATP molecules (34, 35). The transfer from the “H” to the “J” state and the transfer from the “J” to “K” state (Fig. 4) releases about –8 1 kcal/mol in each since it involves a change from (ATP + water) to (ADP + Pi) in aqueous solution for one ATP molecule. The estimates of the energetics of the ATP hydrolysis come from our experience of studying similar molecular machines (22, 33). As we observe in Fig. 5B, the directionality of the system becomes noticeably more prominent (as compared with Fig. 5A), indicating how the electrostatic of the system and the ATP hydrolysis is pushing the system in the functional pathway. A more detailed 2D plot with systematic and hierarchical addition of energy terms is provided in SI Appendix, Fig. S4. We also note that including the CG and the PDLD/S-LRA/ contributions leads to some double counting of the protein/substrate interaction. However, this interaction is usually underestimated by the CG calculations (see SI Appendix, section S3).
The exact mechanism of how the uncorrelated or stepwise ATP hydrolysis activates the conformational changes in the six different Rpt units is one of the most interesting questions regarding this motor system (15). This problem may be resolved using a combination of single-molecule, structural, and computational studies. This is an area that may be the focus of our research in the near future. Such studies should help to unravel the coupling between conformational change and chemistry and to model the time dependence of the system with the kinetic information from the emerging experimental observations.
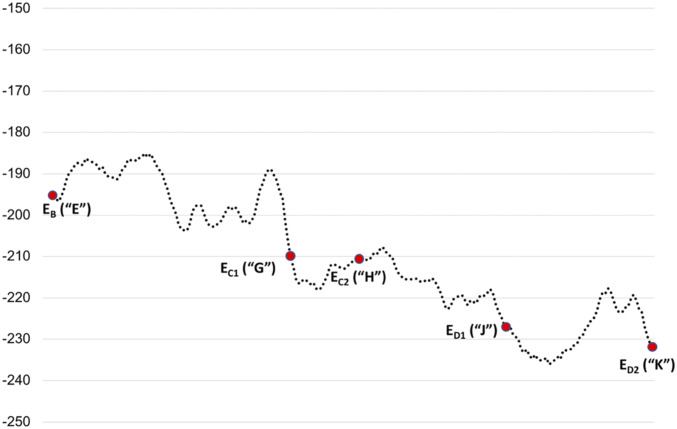
The conformational barriers along the functional pathway were estimated from the CG profiles for the structural conversions (Fig. 6). The intermediate structures that were then used in the CG calculations were generated by steered molecular dynamics (SI Appendix, Fig. S2) simulations. Note that there are significant barriers for going from structure “E” to “G,” where we have a significant structural change. This accounts for the fact that the “E”-to-“G” transition requires two ATP hydrolysis steps (Fig. 4) (20). We also observed reasonable barrier in the conversion of structure “J” to “K.” Conversion of structure “G” to “H” and “H” to “J” does not show any apparent barriers. The entire CG profile shows that the system is moving in the downhill energy direction.
Fig. 6.
The entire CG free energy profile (average on the forward and backward movements) for moving from structure “E” to “K.” Structures EB, EC1, EC2, ED1, and ED2 are designated as structures “E,” “G,” “H,” “J,” and “K” throughout the paper.
Residue Contributions and Mutagenesis Analysis.
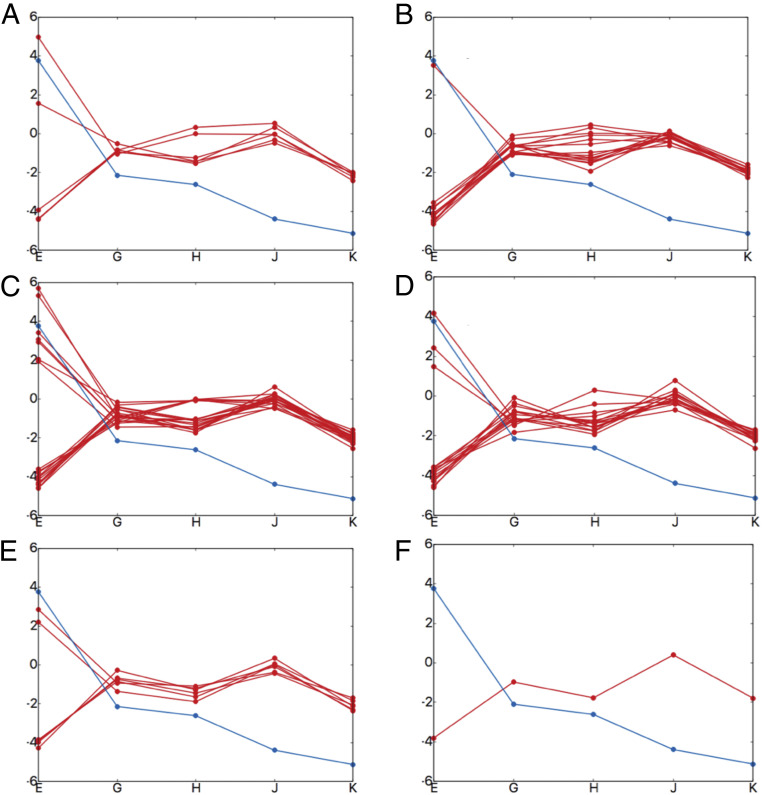
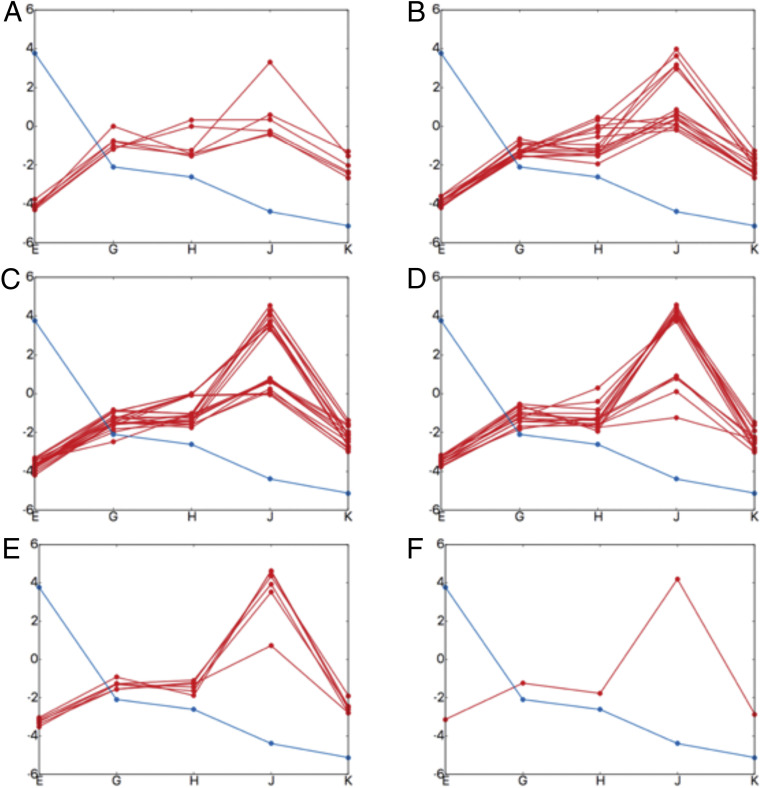
We evaluated the contributions of the key residues for going from structure E to G, G to H, H to J, and J to K, in order to explore their contribution to the landscape. SI Appendix, Figs. S6 and S7 (the arrangement of pore loops around the substrate is shown in SI Appendix, Figs. S8 and S9) show that the bulkier residues in pore loop 1 contribute significantly to the substrate translocation. This is consistent with the statement of Lander and coworkers (15), who proposed that “bulky, aromatic substrate residues intercalate between the pore loop aromatics, akin to the teeth of two cogs, strengthening the grip on the substrate.” In order to validate the importance of these bulkier residues (tyrosine and phenylalanine; see SI Appendix, Fig. S10) we performed computational mutagenesis analysis. As shown in Fig. 7, most of the mutations along pore loop 1 (in different numbers and different combinations) of these bulkier residues disrupt the directional translocation (SI Appendix, Figs. S11–S16 provide details on these mutational calculations). However, our calculations also reveal that the lysine residues prior to the bulkier residues [conserved along pore loop 1 (15)] are also important for the translocation process. This is illustrated in Fig. 8, which shows that all mutation possibilities of these lysine residues disrupt the directional translocation.
Fig. 7.
The effect of mutations on the directional translocation. Each box shows one-dimensional substrate (PDLD/S-LRA/) binding profiles. The x axis denotes the structures (structure EB [“E”], EC1 [“G”], EC2 [“H”], ED1 [“J”], and ED2 [“K”]) and the y axis denotes energy in kilocalories per mole. The blue line shows the wild type and red line shows the mutated profiles (five tyrosine residues and one phenylalanine residue were mutated to alanine). (A) Each red line (six possibilities) shows a single mutation at different position along pore loop 1. (B) Each red line (15 possibilities) shows a combination of two mutations at different positions along pore loop 1. (C) Each red line (20 possibilities) shows a combination of three mutations at different positions along pore loop 1. (D) Each red line (15 possibilities) shows a combination of four mutations at different positions along pore loop 1. (E) Each red line (six possibilities) shows a combination of five mutations at different positions along pore loop 1. (F) The red line (one possibility) shows all the six bulkier residues mutated to alanine along pore loop 1. More details are provided in SI Appendix, Figs. S11–S16.
Fig. 8.
The effect of mutations on the directional translocation. Each box shows one-dimensional substrate (PDLD/S-LRA/) binding profiles. The x axis denotes the structures (structure EB [“E”], EC1 [“G”], EC2 [“H”], ED1 [“J”], and ED2 [“K”]) and the y axis denotes energy in kilocalories per mole. The blue line shows the wild type and the red line shows the mutated profiles (aromatic-prior lysine residues were mutated to alanine). (A) Each red line (six possibilities) shows a single mutation at different positions along pore loop 1. (B) Each red line (15 possibilities) shows a combination of two mutations at different positions along pore loop 1. (C) Each red line (20 possibilities) shows a combination of three mutations at different positions along pore loop 1. (D) Each red line (15 possibilities) shows a combination of four mutations at different positions along pore loop 1. (E) Each red line (six possibilities) shows a combination of five mutations at different positions along pore loop 1. (F) The red line (one possibility) shows all the six lysine residues mutated to alanine along pore loop 1.
Concluding Remarks
The simulations reported in this work reveal that the substrate translocation by the AAA+ motor system of the 26S human proteasome follows a unidirectional motion which is partially guided by the interaction between the substrate and the AAA+ motor protein and the electrostatics of the entire system. To elucidate the effect of the interaction between the substrate and the motor protein we considered the translocation of both a substrate of 13 alanine residues and a substrate containing 12 alanine residues and 1 lysine residue, where the lone lysine residue is present at the front end penetrating through the pore loop. The “lysine substrate” resulted into more pronounced unidirectional motion in our 2D free energy landscape than the nonpolar substrate, suggesting a role for the electrostatic interaction in the translocation process. It is important to note that there is no concrete experimental evidence yet for the electrostatic dominance in proteasomal substrate translocation. Nevertheless, the effect of charge on the degradation rate cannot be ignored since several researchers already exploited the effect of charge in nonhuman proteasomes and observed reasonably accelerated rates of the unfolding and the degradation. This has been true for artificially engineered proteasomes as well, opening a new avenue in drug discovery promoting targeted protein degradation, which is a rapidly emerging novel concept in cancer therapy. We believe that the impact of charges on the translocation of proteasomal substrate, particularly in 26S human proteasome, is yet to be realized and may soon appear as more detailed structural and biophysical studies continue to emerge.
In addition to understanding the effect of the substrate–protein interaction on the directionality of the system, we also examined how the electrostatics of the entire system influence the free energy landscape. We focused on the change while the six Rpt units undergo significant conformational changes, simultaneously pulling and translocating the substrate in a hand-over-hand mechanism. Thus, we used our CG treatment which focuses primarily on the electrostatic terms. It is interesting to note that the directionality of the system could be successfully reproduced by our CG free energy profile without even considering the effect of substrate–protein interactions. This emphasizes the fact that the substrate–protein interaction along the binding region is not the only driving force that helps the substrate penetrate through the narrow channel of the AAA+ motor. Furthermore, the rearrangement of the Rpt units helps in defining the directionality of the electrostatic free energy landscape.
We also performed an analysis of the translocation process by evaluating the electrostatic group contributions (along loops 1 and 2 of the pore) on the translocation potential of the substrate. Our mutagenesis analysis of the one-dimensional free energy profiles strongly supports the experimental observation (by multiple research groups in the literature) that bulkier residues (tyrosine and phenylalanine) along loop 1 are crucially responsible for translocating the substrate along the narrow channel of the AAA+ motor. However, our mutagenesis analysis also reveals that the conserved lysine residues (prior to bulkier residues) are also important in substrate translocation. Finally, we simulated the translocation trajectory connecting the five substrate-bound AAA+ states and evaluated the conformational barriers in different steps of the translocation process. Our CG energy profile suggests that multiple significant barriers are involved on going from state EB (“E”) to EC1 (“G”), explaining why two ATP hydrolysis events are required to make this step happen. Thus, we believe that in this paper we were able to develop a systematic and consistent analysis of structure-based molecular description of the energetics of the translocation process of 26S human proteasome. Our analysis has the potential to reveal general principles in the underlying physics of the translocation directionality in AAA+ motor types across several species.
Materials and Methods
In this paper we have used the PDLD/S-LRA/ (23) method for the calculations of binding free energies of the substrate. To construct the 2D free energy landscape we also used our CG model, which calculates the total energy of the system. A brief overview of these two methods is given below. Details of these methods can be found in ref. 23 (PDLD/S-LRA/) and ref. 30 (CG model). The MOLARIS-XG package (36) was used for all the PDLD/S-LRA/ and CG free energy calculations. For construction of the substrate-bound AAA+ motor system, Rpt 1 to 6 and 13 residues of the substrate were extracted from the entire structure (Figs. 1 B and C and 3).
PDLD/S-LRA/β.
To compute the binding free energies of the substrate, the scaled semimacroscopic PDLD method was used with the LRA treatment with a scaled nonelectrostatic term (PDLD/S-LRA/) (23). Our PDLD/S-LRA/ method can estimate binding energies effectively by constructing proper thermodynamic cycles. The PDLD represents water molecules by Langevin dipoles. The energy is evaluated using the linear response approximation, which averages the energy of the charged and uncharged solute. A relaxation run (0.1 ns) was performed before all binding free energy calculations.
Total Energy Calculation through the CG Model.
Our CG model is consistently being upgraded, focusing on the electrostatics of the proteins and their relationship with the solvation of ionizable residues. The total energy of the system is calculated in this model through the following equation:
where is the main-chain explicit contribution that includes torsional terms, is the electrostatic free energy, is the hydrophobic solvation energy, is the hydrophilic (polar) solvation energy, is the effective van der Waals free energy, and is the effective hydrogen bond free energy. Before performing CG energy evaluation we also apply a Monte Carlo proton transfer (MCPT) method to evaluate the ionization states of all of the ionizable residues. In the MCPT approach (37), the MC controls proton transfer between ionizable residues or between one ionizable residue and the bulk. The acceptance probability of the move is determined by standard Metropolis criteria. The actual MCPT can be used for time-dependent study of proton transport processes. However, here we use this approach just to obtained equilibrated ionization states. After the MCPT procedure the CG energies are evaluated by the total energy equation.
Supplementary Material
Acknowledgments
This work was supported by NSF Grant MCB 1707167 and NIH Grant R35 GM122472. We thank the University of Southern California High Performance Computing and Communication Center, as well as the Extreme Science and Engineering Discovery Environment’s Comet facility at the San Diego Supercomputing Center, for computational resources.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2104245118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Marques A. J., Palanimurugan R., Matias A. C., Ramos P. C., Dohmen R. J., Catalytic mechanism and assembly of the proteasome. Chem. Rev. 109, 1509–1536 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Serrano-Aparicio N., Świderek K., Moliner V., Theoretical study of the inhibition mechanism of human 20S proteasome by dihydroeponemycin. Eur. J. Med. Chem. 164, 399–407 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Saha A., Oanca G., Mondal D., Warshel A., Exploring the proteolysis mechanism of the proteasomes. J. Phys. Chem. B 124, 5626–5635 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciechanover A., Heller H., Elias S., Haas A. L., Hershko A., ATP-dependent conjugation of reticulocyte proteins with the polypeptide required for protein degradation. Proc. Natl. Acad. Sci. U.S.A. 77, 1365–1368 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hershko A., Ciechanover A., Heller H., Haas A. L., Rose I. A., Proposed role of ATP in protein breakdown: Conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc. Natl. Acad. Sci. U.S.A. 77, 1783–1786 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie S. C., et al., The structure of the PA28-20S proteasome complex from Plasmodium falciparum and implications for proteostasis. Nat. Microbiol. 4, 1990–2000 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Löwe J., et al., Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 A resolution. Science 268, 533–539 (1995). [DOI] [PubMed] [Google Scholar]
- 8.Majumder P., et al., Cryo-EM structures of the archaeal PAN-proteasome reveal an around-the-ring ATPase cycle. Proc. Natl. Acad. Sci. U.S.A. 116, 534–539 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Javidialesaadi A., Stan G., Asymmetric conformational transitions in AAA+ biological nanomachines modulate direction dependent substrate protein unfolding mechanisms. J. Phys. Chem. B 121, 7108–7121 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Javidialesaadi A., Flournoy S. M., Stan G., Role of diffusion in unfolding and translocation of multidomain titin I27 substrates by a Clp ATPase nanomachine. J. Phys. Chem. B 123, 2623–2635 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y., et al., Structural mechanism for nucleotide-driven remodeling of the AAA-ATPase unfoldase in the activated human 26S proteasome. Nat. Commun. 9, 1360 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogura T., Wilkinson A. J., AAA+ superfamily ATPases: Common structure–Diverse function. Genes Cells 6, 575–597 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Neuwald A. F., Aravind L., Spouge J. L., Koonin E. V., AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27–43 (1999). [PubMed] [Google Scholar]
- 14.Erzberger J. P., Berger J. M., Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu. Rev. Biophys. Biomol. Struct. 35, 93–114 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Puchades C., Sandate C. R., Lander G. C., The molecular principles governing the activity and functional diversity of AAA+ proteins. Nat. Rev. Mol. Cell Biol. 21, 43–58 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer L. M., Leipe D. D., Koonin E. V., Aravind L., Evolutionary history and higher order classification of AAA+ ATPases. J. Struct. Biol. 146, 11–31 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Monroe N., Hill C. P., ATPases: Protein polymer disassembly machines. J. Mol. Biol. 428, 1897–1911 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frickey T., Lupas A. N., Phylogenetic analysis of AAA proteins. J. Struct. Biol. 146, 2–10 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Livneh I., Cohen-Kaplan V., Cohen-Rosenzweig C., Avni N., Ciechanover A., The life cycle of the 26S proteasome: From birth, through regulation and function, and onto its death. Cell Res. 26, 869–885 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong Y., et al., Cryo-EM structures and dynamics of substrate-engaged human 26S proteasome. Nature 565, 49–55 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de la Peña A. H., Goodall E. A., Gates S. N., Lander G. C., Martin A., Substrate-engaged 26S proteasome structures reveal mechanisms for ATP-hydrolysis-driven translocation. Science 362, eaav0725 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H., Shi Y., Chen X. S., Warshel A., Simulating the electrostatic guidance of the vectorial translocations in hexameric helicases and translocases. Proc. Natl. Acad. Sci. U.S.A. 106, 7449–7454 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh N., Warshel A., Absolute binding free energy calculations: On the accuracy of computational scoring of protein-ligand interactions. Proteins 78, 1705–1723 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F., et al., Mechanism of substrate unfolding and translocation by the regulatory particle of the proteasome from Methanocaldococcus jannaschii. Mol. Cell 34, 485–496 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fei X., et al., Structures of the ATP-fueled ClpXP proteolytic machine bound to protein substrate. eLife 9, e52774 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudriaeva A., Kuzina E. S., Zubenko O., Smirnov I. V., Belogurov A. Jr., Charge-mediated proteasome targeting. FASEB J. 33, 6852–6866 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Azuma Y., Bader D. L. V., Hilvert D., Substrate sorting by a supercharged nanoreactor. J. Am. Chem. Soc. 140, 860–863 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pu J., Karplus M., How subunit coupling produces the gamma-subunit rotary motion in F1-ATPase. Proc. Natl. Acad. Sci. U.S.A. 105, 1192–1197 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koga N., Takada S., Folding-based molecular simulations reveal mechanisms of the rotary motor F1-ATPase. Proc. Natl. Acad. Sci. U.S.A. 103, 5367–5372 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messer B. M., et al., Multiscale simulations of protein landscapes: Using coarse-grained models as reference potentials to full explicit models. Proteins 78, 1212–1227 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levitt M., Warshel A., Computer simulation of protein folding. Nature 253, 694–698 (1975). [DOI] [PubMed] [Google Scholar]
- 32.Vicatos S., Roca M., Warshel A., Effective approach for calculations of absolute stability of proteins using focused dielectric constants. Proteins 77, 670–684 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee S., Warshel A., Electrostatic origin of the mechanochemical rotary mechanism and the catalytic dwell of F1-ATPase. Proc. Natl. Acad. Sci. U.S.A. 108, 20550–20555 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strajbl M., Shurki A., Warshel A., Converting conformational changes to electrostatic energy in molecular motors: The energetics of ATP synthase. Proc. Natl. Acad. Sci. U.S.A. 100, 14834–14839 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber J., Senior A. E., Catalytic mechanism of F1-ATPase. Biochim. Biophys. Acta 1319, 19–58 (1997). [DOI] [PubMed] [Google Scholar]
- 36.Warshel A., et al., Molaris-XG, v 9.15 (University of Southern California, Los Angeles, 2012).
- 37.Vorobyov I., Kim I., Chu Z. T., Warshel A., Refining the treatment of membrane proteins by coarse-grained models. Proteins 84, 92–117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.