Abstract
Evolutionary theory suggests that commonly found sex differences are largest in healthy populations and smaller in populations that have been exposed to stressors. We tested this idea in the context of men’s typical advantage (vs. women) in visuospatial abilities (e.g., mental rotation) and women’s typical advantage (vs. men) in social-cognitive (e.g., facial-expression decoding) abilities, as related to frequent binge drinking. Four hundred nineteen undergraduates classified as frequent or infrequent binge drinkers were assessed in these domains. Trial-level multilevel models were used to test a priori Sex × Group (binge drinking) interactions for visuospatial and social-cognitive tasks. Among infrequent binge drinkers, men’s typical advantage in visuospatial abilities and women’s typical advantage in social-cognitive abilities was confirmed. Among frequent binge drinkers, men’s advantage was reduced for one visuospatial task (Δ d = 0.29) and eliminated for another (Δ d = 0.75), and women’s advantage on the social-cognitive task was eliminated (Δ d = 0.12). Males who frequently engaged in extreme binges had exaggerated deficits on one of the visuospatial tasks, as did their female counterparts on the social-cognitive task. The results suggest sex-specific vulnerabilities associated with recent, frequent binge drinking, and support an evolutionary approach to the study of these vulnerabilities.
Keywords: sex differences, sexual selection, alcohol, binge drinking, cognitive deficits, vulnerabilities
Introduction
Human sex differences in various cognitive domains are well established (Archer, 2019; Geary et al., 2000; Hall & Matsumoto, 2004; McClure, 2000; van Beek & Dubas, 2008; Voyer et al., 2017), but their origins are vigorously debated. The finding that the magnitude of many of these sex differences varies across contexts has been interpreted as evidence for environmental and not biological origins (Asperholm et al., 2019). However, an evolutionary perspective can be used to predict and understand these contextual influences while placing them in the same unifying framework used to study sex differences in nonhuman species, that is, sexual selection (Andersson, 1994; Darwin, 1871). Sexual selection involves competition for mates or other reproductively important resources and discriminative mate choice, all of which result in the exaggeration of the associated traits and an emergence of sex differences in those traits (see Geary, 2021, for an extensive review).
Critically, these traits are condition dependent—their development and expression are reliable indicators of the individuals’ exposure and resilience to ecological (e.g., parasite-induced) and social (e.g., physical combat) stressors (Cotton et al., 2004; Geary, 2015; Johnstone, 1995; Zahavi, 1975). For example, the peacocks’ (Pavo cristatus) train is an elaborated sex-specific trait that appears to signal immune system health (Loyau et al., 2005); the condition of male health is directly reflected in the length and attractiveness of the train. One result is that any associated sex differences are largest in well-nourished populations that are buffered from exposure to ecological and social stressors. As conditions deteriorate, many members of the advantaged sex can no longer build and maintain exaggerated traits and thus the magnitude of any associated sex differences becomes smaller (see Figure 1). These same traits also appear to be more sensitive to man-made toxins than are other traits (Bortolotti et al., 2003; Geary, 2015, 2019; Jašarević et al., 2011). Nearly all of the prior research on condition-dependent trait expression has been conducted with nonhuman species, including arthropods, birds, fish, and mammals (see Geary, 2015). For example, antler size in red deer (Cervus elaphus) and beak color in American goldfinches (Spinus tristis) are condition-dependent traits in males and females, respectively (Geary, 2015). Here, we expand this work to humans and test the prediction that sex differences found in healthy populations will be smaller in populations that have been exposed to a potential neurotoxin, operationalized here as exposure to ethyl alcohol through frequent heavy episodic drinking (i.e., binge drinking; e.g., Hindmarch et al., 1991).
Figure 1.

Simulated data demonstrating larger sex differences in healthy populations (top distributions) and smaller sex differences in populations with exposure to stressors, with stronger effects of stressors on the advantaged sex (bottom distributions).
Condition-Dependent Traits in Humans
Sex differences in physical size, strength, rate of development, and lifespan are consistent indicators of an evolutionary history of sexual selection in mammals (e.g., Clutton-Brock & Isvaran, 2007). These same sex differences are found in humans and are consistent with an evolutionary history of male-male competition in our species (Leigh, 1995; Tanner, 1990). Although the research in this area has not been framed in terms of condition dependence, one supporting example is that men’s advantage in height is largest in healthy populations with access to modern healthcare, and becomes smaller in populations exposed to chronic stressors (Perkins et al., 2016). In traditional societies (i.e., hunter-gatherer and some horticulture societies; Geary, 2021), in addition to physical contests, competition among men involves larger travel ranges and use of projectile weapons, both of which are supported by different aspects of visuospatial ability (e.g., MacDonald & Hewlett, 1999). In such contexts, men with larger ranges perform better than other men on standard visuospatial tasks, and have more wives and children (Vashro & Cashdan, 2015). The same male advantage in visuospatial skills is found in developed nations (Voyer et al., 1995).
As with other primates, competition among women over valuable resources is common (Smuts, 1987; Stockley & Campbell, 2013). The ethnographic record and studies in developed nations indicate that this competition is more likely to manifest as relational rather than physical aggression (Card et al., 2008; Jankowiak et al., 2005). Relational aggression involves of the use of social competencies to undermine the relationships of competitors, including those with potential mates. In traditional contexts, socially dominant women often have healthier and more surviving children than do subordinate ones (e.g., Ji et al., 2013). Women’s competitiveness appears to be enhanced by various social-cognitive abilities, including sensitivity to nonverbal cues and facial expressions, especially the facial expressions of other women. These skills, in turn, contribute to women’s advantage—relative to men—in making inferences about the thoughts and feelings of others (i.e., theory of mind; Geary et al., 2014). Women’s advantages (relative to men) in these and related areas are well documented (e.g., Hall, 1984; Thompson & Voyer, 2014) and evident across cultures in South America, North America, Southern Europe, and Central Europe (Merten, 2005). One potential exception is men’s heightened sensitivity (relative to women) to the angry expressions of other men, which functions as a common social signal in men’s dominance-related conflicts (Rotter & Rotter, 1988).
Binge Drinking and Cognitive Deficits
In natural contexts, ripe and decaying fruit often promote fermentation and the production of ethanol. Ethanol increases the caloric value of the fruit and produces an odor that can aid in its location. Fruit eating species, including many primates, often ingest such fruits and exhibit intoxication-related behaviors soon thereafter (Dudley, 2000). Dudley proposed that the caloric gains from eating such fruits may be the evolutionary basis for the reward value of ethanol and ungirds the risk of Alcohol Use Disorder (AUD). From this perspective, AUDs reflect a “maladaptive co-option” of once advantageous consumption of ripe and decaying fruit containing ethanol (Dudley, 2000; Nesse, 2002). Alcohol use can quickly become maladaptive because it is now more readily available and is more potent, pure, and easily administered than in natural contexts (Dudley, 2014). The result is that heavy drinking, particularly in the context of AUD, becomes a neurotoxic stressor—one with potentially sex-specific neurobehavioral consequences (Nixon et al., 2014).
Predictions from Geary (2015) specify that sex differences in sexually selected cognitive abilities should be present in groups of people exposed to few stressors or toxins, whereas diminished sex differences should be apparent in groups of people exposed to more stressors or toxins. Cognitive deficits associated with AUD—including deficits in visuospatial and social-cognitive abilities—have been well established (Bora & Zorlu, 2017; Fama, 2019, Parsons & Nixon, 1993; Rourke & Grant, 2009; Sullivan, 2017). Though there is some evidence that heavy alcohol abuse results in greater impairment of women’s social-cognitive abilities (compared to men; Valmas et al., 2014), and that alcoholic men experience greater impairment in visuospatial abilities (compared to alcoholic women; Sullivan et al., 2000, 2002), associated sex differences remain largely unexplored (Geary, 2017).
In addition to heavy drinking in the context of AUD, recent (i.e., past month) and frequent binge drinking—which is characterized by drinking five or more drinks for men (four or more drinks for women) in about two hours (NIAAA, 2004)—also can be considered a neurotoxic stressor (Carbia et al., 2018; Jacobus & Tapert, 2013; Lannoy et al., 2019). Indeed, it has been suggested that as little as one binge drinking episode could cause at least temporary cognitive deficits among humans, as it does in rats (Obernier et al., 2002). For example, past-year number of drinking days and past three-month drinking was related to deficits in neuropsychological functioning in a prospective study of adolescents (Squeglia et al., 2009); past 6-month binge drinking was related to cognitive performance among young adults (Townshend & Duka, 2005); and previous-evening binge drinking was associated with compromised attentional control and mood among college students (Howland et al., 2010).
There is some preliminary evidence that male binge drinkers experience greater impairment in visuospatial abilities (compared to females; Hartley et al., 2004), and that female binge drinkers experience greater impairments in social-cognitive abilities (compared to males; Carbia et al., 2018; Lannoy et al., 2018), but any such sex differences have not been systematically explored, much less assessed in a unifying evolutionary framework. Here, we propose that many cognitive deficits associated with recent, frequent binge drinking might be nuanced in sex-specific ways.
Current Study
The current study tested the hypothesis that men’s advantage in visuospatial abilities and women’s advantage in social-cognitive abilities will show the same pattern of condition-dependent expression found for sexually selected traits in nonhuman species (Cotton et al., 2004; Johnstone, 1995; Zahavi, 1975). To test this hypothesis in the context of recent, frequent binge drinking, we used two standard measures of visuospatial abilities and developed a facial-expression decoding task as a measure of social-cognitive ability (see Hone, Scofield, Bartholow, & Geary, 2019). We expected advantages for men on the visuospatial tasks and an advantage for women on the social-cognitive task among emerging adults who do not drink alcohol or who are infrequent binge drinkers, and attenuated sex differences among more frequent binge drinkers. To control for potential group differences in overall cognitive ability, we administered a vocabulary test that typically does not show sex differences and is a reliable measure of general intelligence (Jensen, 1998).
Method
Participants
Participants were 429 undergraduates recruited from Introductory Psychology courses at a large, public, Midwestern University between 2016 and 2019. Demographic survey data revealed that 95% of participants were non-Hispanic and 5% were Hispanic or Latino/a/x. The racial composition of this sample was 83.3% White, 7.4% Black/African American, 6.7% Asian, 1% American Indian/Alaska Native, with the remaining unknown. Additionally, 34.4% of participants reported being a member of a Greek fraternity or sorority.
Eight participants who did not complete the vocabulary test and two participants who failed to follow experimental instructions were excluded from analyses, leaving 419 individuals (N = 233 women, age: M = 18.61, SD = 0.85; N = 186 men, age: M = 19.26, SD = 1.52) in the sample. Of these individuals, those who completed one (N = 175; 84 women) or another (N = 209; 125 women) visuospatial task were included in the analyses, with the exception of 35 individuals whose performance on the first visuospatial task was below chance. All 419 participants contributed data to analyses of the social-cognitive task.
Participants were recruited from Introductory Psychology courses. Initially, 210 individuals were recruited regardless of their recent binge drinking status; these participants completed past-month binge drinking frequency measures in the lab. Subsequently, to ensure representation across the spectrum of recent binge drinking frequency, 209 additional participants were recruited based on their responses to past-month binge drinking items administered as part of an online mass screening survey. For this part of the sample, we targeted individuals who reported binge drinking infrequently (or not at all) in the past month, as well as individuals who reported binge drinking relatively frequently within the past month (Townshend & Duka, 2005).
As this was the first a priori study of its kind, expected interaction effect sizes were unknown. However, Stavro et al. (2012) estimated alcohol-related visuospatial deficits to range from d of 0.49–0.59. Thus, an a priori power analysis was performed assuming medium (ηp2 = .06; Cohen’s f = 0.25) interaction effects (cf. Simonsohn, 2014 1 ). To achieve 80% power using a four-group contrast, a sample size of 45 individuals per group (180 total) was suggested. We biased participant recruitment to meet and exceed this sample size suggestion to ensure an adequately powered study.
Measures
Vocabulary test
The Vocabulary Test II was included as a measure of general intelligence and used as a control variable (Ekstrom et al., 1976). The test includes 36 vocabulary words and requires participants to choose the synonym of a target word from among five options (e.g., target vocabulary word “edifice,” with synonym options of “small insect,” “heir,” “front,” “large building,” and “learning”). The score is the number of correct synonyms selected out of 36 (M = 21.10, SD = 3.91, α = .79).
Recent binge drinking frequency
We assessed frequency of binge drinking in the past month using items compiled by the Multi-disciplinary Alcoholism Research Center (MARC) at the University of Missouri, which have been used in numerous previous studies. Specifically, we administered three items measuring different facets of recent binge drinking (e.g., Martins et al., 2018): (1) a regular binge, “In the past 30 days, how many times have you had five or more drinks in a single sitting?”; (2) an extreme binge, “In the past 30 days, how many times have you had 12 or more drinks at a single sitting?”; and (3) a binge with a more stringent time-frame likely to quickly produce an intoxicating blood alcohol concentration, “During the last 30 days, how often did you have 5 or more (males) or 4 or more (females) drinks containing any kind of alcohol within a two-hour period?” Responses were made using an eight-point scale ranging from 0 (“Did not have ___ or more drinks in ___,”) to 8 (“twice a day or more”), and were recoded to a frequency per week scale. As binge drinking items were positively correlated (rs = .50 to .80, ps < .001), scores were aggregated to create a composite binge drinking frequency variable (α = .89; McDonald’s ω = .86), in line with prior work (e.g., Martins et al., 2018).
The distribution of scores on the binge drinking frequency composite variable was heavily positively skewed (see Figure 2), with roughly half of the respondents reporting little-to-no binge drinking in the past month, and the other half of the respondents distributed in a long “tail” with varying frequencies of past-month binge drinking. Thus, from the composite binge drinking frequency score we created two groups of participants (see Table 1 and Figure 2): an infrequent binge drinking group (N = 210; M = 0.09, SD = 0.19) and a frequent binge drinking group (N = 209; M = 2.93, SD = 2.51). These groups were formed on the basis of a two-quantile split dictated from the range of binge drinking frequency composite scores (this is equivalent to a median-split; see the Hmisc package in R). While this binning procedure could result in a decrease in statistical power (cf. Royston et al., 2005), it is not without precedent (e.g., Courtney & Polich, 2010; Maurage et al., 2012). Further, data from the infrequent binge drinking group were critically used to confirm whether the visuospatial and social-cognitive measures were capturing the expected sex differences, whereas data from the frequent binge drinking group permitted tests of the prediction that the magnitude of these sex differences will be smaller following stressor exposure.
Figure 2.
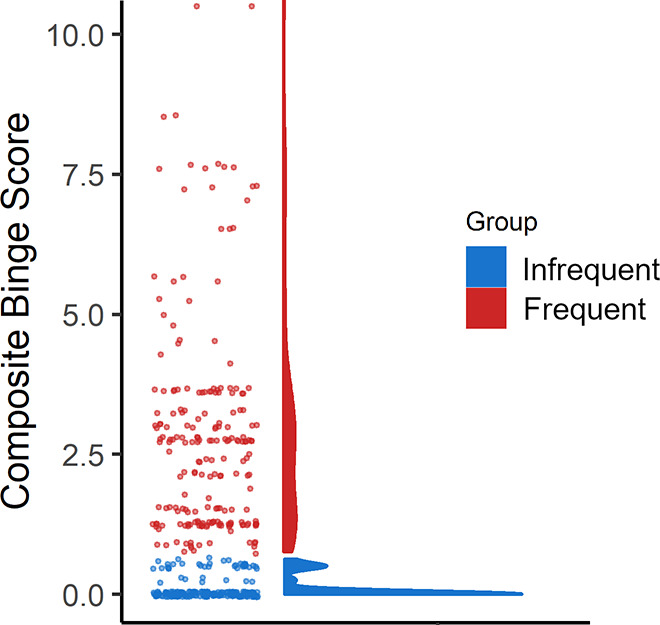
Composite binge drinking scores for the infrequent (blue) and frequent (red) binge drinking groups based on a two-quantile split, and a density plot showing the skewed distribution of binge drinking scores across all groups.
Table 1.
Past 30-day Binge Drinking Frequency (per week) Descriptive Statistics.
| Measure | Infrequent | Frequent |
|---|---|---|
| How many times have you had five or more drinks in a single sitting? | 0.05 (0.11) | 1.52 (1.07) |
| How often did you have 5 or more (males) or 4 or more (females) drinks containing any kind of alcohol within a two-hour period? | 0.05 (0.11) | 1.12 (1.26) |
| How many times have you had twelve or more drinks at a single sitting? | 0.001 (0.02) | 0.29 (0.64) |
| Composite binge drinking frequency | 0.09 (0.19) | 2.93 (2.51) |
Note: Mean values (SD) for the infrequent and frequent binge drinking groups are presented for the three binge drinking frequency measures as well as the binge drinking frequency composite. The values represent binge episodes per week during the past 30 days.
Visuospatial ability
The Mental Rotation Test (MRT) involves the three-dimensional mental rotation of 24 geometric figures presented on a computer monitor (Peters et al., 1995). In each trial, a target geometric figure is presented along with four response options depicting figures rotated to various degrees. Participants are required to identify the two out of four response options that correctly depict the target figure. The trial ends when participants indicate their second response by pressing a corresponding button, and the next trial begins immediately thereafter. Scoring on each trial was based on whether participants correctly identified both rotations of the target figure. Reliability estimates based on response accuracy (M = 0.60, SD = 0.23, α = .92) and response time (M = 25.89 s, SD = 8.14, α = .95) were excellent.
The MRT was administered via computer. Typically, this task is administered via pencil and paper and participants work at their own pace. To ensure that our modifications were not affecting performance, we assessed its psychometric properties and whether it was capturing the expected sex difference among the first 175 participants. Analyses of MRT performance confirmed the expected male advantage, but a relatively large number of participants (N = 35) exhibited chance levels of performance (i.e., < 5 correct out of 24 trials). Due to the difficulty of the MRT and to mitigate chance performance, we subsequently replaced the task with a less difficult one, the Judgment of Line Angle and Position Test (JLAP; Collaer & Nelson, 2002).
The JLAP Test involves comparing the angle of a single target line to the angles of 15 comparison line options presented in an array, as shown in Figure 3 (Collaer & Nelson, 2002). In each of 20 trials, a target line is randomly selected from the 15 line options, and participants must identify which of the options matches the angle of the target line. The trial ends when the participant indicates using the computer mouse which of the 15 lines matches the target line, after which the next trial begins. Accuracy precision (distance between the response option and the target line) and response times are recorded from each trial. Better performance is indicated by a smaller difference between the angle of the chosen option and that of the target line (i.e., less negative value). Precision reliability was modest (M = −0.33, SD = 0.35, α = .57), but response time reliability was very good (M = 3.85 s, SD = 0.65, α = .87).
Figure 3.
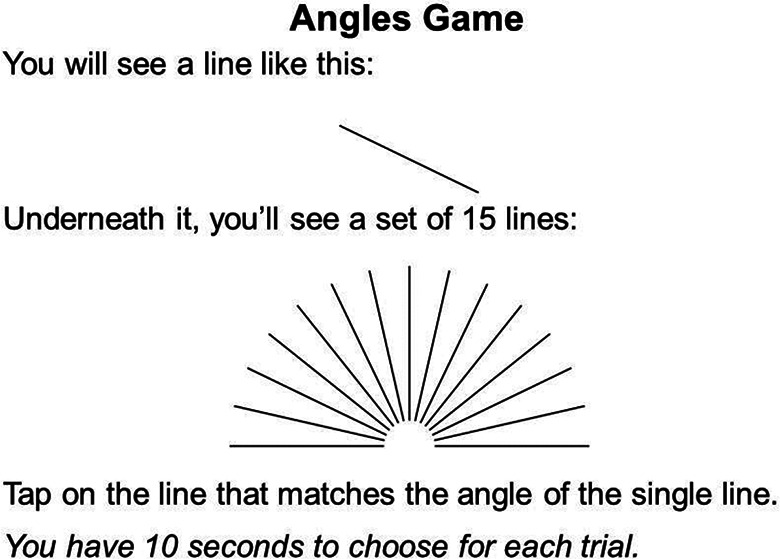
Example item from the Judgment of Line Angle and Position (JLAP) test.
Social-cognitive ability
The Facial-Expression Decoding Task (Hone et al., 2019) assesses the speed and accuracy of emotion recognition and was used as a measure of social-cognitive ability. The task involves identifying the emotion being expressed in each of 34 faces, which begin as highly pixelated objects and then slowly come into focus. The images were selected from freely available face image databases (see supplemental materials; https://osf.io/r7b5x). Each face is presented within a movie file comprising 78 frames that range from completely obscured to completely clear with regard to pixelated noise. Frames are separated by a 600 ms delay, yielding stimulus “movies” that are approximately 45 s long. The 34 stimulus movie files (17 male; 17 female) each portray faces displaying one of seven emotions (happy, angry, surprise, fear, sad, disgust, neutral). Participants’ task on each trial is to press a key once they recognize the emotion being displayed on the face, which halts the movie and replaces the face image with a visual mask (500 ms duration). Following the mask, a screen listing the seven emotion response options is displayed and the participant chooses the displayed emotion using the keyboard number pad (numbers 1–7).
The next trial begins immediately after the participant responds. Across the task, each of the seven emotions is presented at least four times, with at least two male faces and at least two female faces displaying each emotion. In this study, a subsample of participants (N = 230) was presented with two additional angry male faces. Primary variables include facial-expression decoding accuracy (M = 0.70, SD = 0.09, α = .58) and response time (M = 2.18 s, SD = 0.41, α = .83). The facial-expression decoding task was developed expressly for this study. Thus, as with the MRT, we assessed whether it was capturing the expected sex difference (it was); see Hone et al. (2019) for a discussion of the validity of the facial-expression decoding task.
Procedure
This study was approved by the University of Missouri’s Institutional Review Board (# 2003561) and written informed consent was obtained from all participants. The study protocol was carried out in accordance with the provisions of the World Medical Association Declaration of Helsinki. Participants were invited to participate in a 90-minute laboratory session for course credit. Upon arrival, a research assistant obtained informed consent and the participant was seated at a computer station. Participants first completed one of the visuospatial tasks and the facial-expression decoding task in a randomized order, after which they completed the vocabulary test and alcohol use measures, in a randomized order. Subjects were instructed to respond to the cognitive tasks as quickly and as accurately as possible. The cognitive tasks were programmed using E-Prime software (Psychology Software Tools, 2016), and the questionnaire measures were administered using Qualtrics software (https://www.qualtrics.com).
Analyses
Trial-level multilevel modeling was used to assess sex and group (past-month binge drinking frequency) differences in visuospatial abilities and facial-expression decoding, nesting trials within subjects and specifying random intercepts. A common model building procedure was used to specify the multilevel models. First, a maximal model (including all potential random effects) was fit. Random effects were then iteratively dropped, and the simplest model without decreases in model fit was retained, which in our case specified only random intercepts, nesting trials within subjects (Matuschek et al., 2017). The vocabulary score was included as a covariate in all models, and the homogeneity of regression slopes assumption was retained across all tasks for the accuracy (ps ≥ .81) and response time (ps ≥ .11) measures. Models assessing accuracy additionally controlled for individual variation in response times.
Sex × Group interactions were tested by specifying interaction contrasts, following guidelines from Rosnow and Rosenthal (1995). This entails testing the group means for the four conditions as a one-way ANOVA (infrequent binge drinking men, frequent binge drinking men, infrequent binge drinking women, and frequent binge drinking women) against the predicted pattern of means for sex difference attenuations (infrequent binge drinking advantaged sex: +2, frequent binge drinking advantaged sex: +1, infrequent binge drinking disadvantaged sex: −2, frequent binge drinking disadvantaged sex: −1), afforded by a priori predictions (Geary, 2015). Post-hoc comparisons (multilevel two-group comparisons) were used to examine sex differences for each binge drinking group separately. That is, we first confirmed expected sex differences in accuracies or response times for the visuospatial tasks and social-cognitive task in the infrequent binge drinking group. Then, for measures exhibiting sex differences, we predicted and tested for the attenuation, elimination, or reversal of sex differences (i.e., decreases in effect size) in the frequent binge drinking group. The attenuation is represented in the interaction contrast. Finally, to assess potential dose-response effects we examined the relation between the frequency of 2-hour binge episodes and extreme binge episodes and task outcomes within the frequent binge drinking group, separately for men and women.
All analyses were performed in R, using the lme4 package. The experiment reported here was not formally pre-registered but the core hypotheses regarding sex-specific vulnerabilities in visuospatial and social-cognitive abilities were developed and reviewed prior to the study (Geary, 2015, pp. 231−265; Geary, 2019; Figure 7). De-identified data and experimental code can be found online at https://osf.io/r7b5x.
Results
Trial-level data (based on response times as compared to subject-averaged accuracy) were first screened for outliers. Seventy-one out of 4,177 trials on the MRT (0.02%), and 30 out of 4,230 trials on the JLAP Test (0.01%) were identified as outliers (> 3 SD from the mean of the scaled response time data; Tabachnik & Fidell, 2012) and were removed from further analysis. On the facial-expression decoding task, 372 out of 19,648 response time trials were marked as outliers (0.02%), and similarly were removed from further analysis. Table 2 includes descriptive statistics for the vocabulary test and cognitive measures.
Table 2.
Descriptive Statistics.
| Sex | Vocabulary Test | Response Time (s) | Accuracy/Precision | |
|---|---|---|---|---|
| MRT | Women | 21.21 (3.96) | 26.11 (8.08) | 0.55 (0.22) |
| Men | 20.87 (4.07) | 25.69 (8.23) | 0.65 (0.23) | |
| JLAP | Women | 21.06 (3.73) | 3.83 (0.65) | −0.35 (0.33) |
| Men | 20.86 (3.99) | 3.88 (0.65) | −0.31 (0.39) | |
| Facial-Expression Decoding |
Women | 20.99 (3.82) | 2.14 (0.39) | 0.70 (0.09) |
| Men | 21.25 (4.03) | 2.24 (0.42) | 0.70 (0.09) |
Vocabulary
As noted, vocabulary was used as a covariate to control for the potential influence of intelligence on task performance. Ancillary analyses indicated that infrequent binge drinkers (M = 21.50, SD = 3.83) had slightly higher vocabulary scores than frequent binge drinkers (M = 20.70, SD = 3.97), F(1,415) = 4.94, p = .027, d = 0.20. There were, however, no sex differences in vocabulary scores, F(1, 415) = 0.46, p = .498, nor was there a significant interaction between sex and binge drinking status, F(1, 415) = 0.49, p = .483. In the infrequent binge drinking group, men (M = 21.57, SD = 4.28) and women (M = 21.46, SD = 3.56) had similar vocabulary scores, F(1,208) = 0.04, p = .838. Likewise, there was no significant difference between men’s (M = 21.02, SD = 3.86) and women’s (M = 20.36, SD = 4.07) vocabulary scores in the frequent binge drinking group, F(1,207) = 1.44, p = .232. Thus, any sex differences on the visuospatial and social-cognitive tasks are unlikely to be related to general cognitive ability.
Mental Rotation Test (MRT)
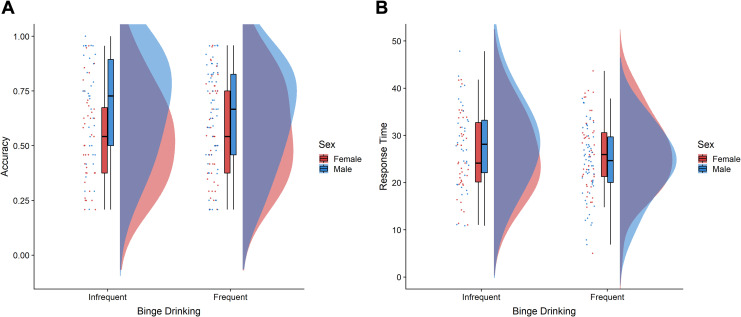
Figure 4 shows mean values for both accuracy and response times for the MRT. The a priori interaction contrast between sex and binge drinking (infrequent binge drinking men: +2, frequent binge drinking men: +1, infrequent binge drinking women: −2, frequent binge drinking women: −1) was significant for accuracy, F(1,163.43) = 12.16, p = .001, η2 = .07. Planned comparisons showed that infrequent binge drinking men (M = 0.68, SD = 0.47) were more accurate than infrequent binge drinking women (M = 0.53, SD = 0.50), F(1,66.28) = 8.58, p = .005, d = 0.72. Men were also more accurate than women in the frequent binge drinking group (Men: M = 0.62, SD = 0.49; Women: M = 0.56, SD = 0.50, F(1,96.07) = 4.47, p = .037), but the magnitude of their advantage (d = 0.43) was smaller than that observed in the infrequent binge drinking group. The interaction contrast for response times (infrequent binge drinking men: −2, frequent binge drinking men: −1, infrequent binge drinking women: +2, frequent binge drinking women: +1) was not significant, F(1,169.36) = 0.50, p = .480.
Figure 4.
Mean values for (A) accuracy and (B) response times (seconds) by sex and binge drinking group for the mental rotation test (MRT).
As predicted, within the frequent binge drinking group, neither the frequency of binge episodes (> 4 drinks), r(43) = −.13, p = .389, nor the frequency of extreme binge episodes (> 11 drinks), r(43) = −.13, p = .381, was related to women’s accuracy on the MRT. The frequency of men’s binge episodes was not related to MRT accuracy, r(55) = .01, p = .957, but a higher frequency of extreme binge episodes was associated with lower accuracy among men, r(55) = −.32, p = .014.
Judgment of Line Angle and Position (JLAP) Test
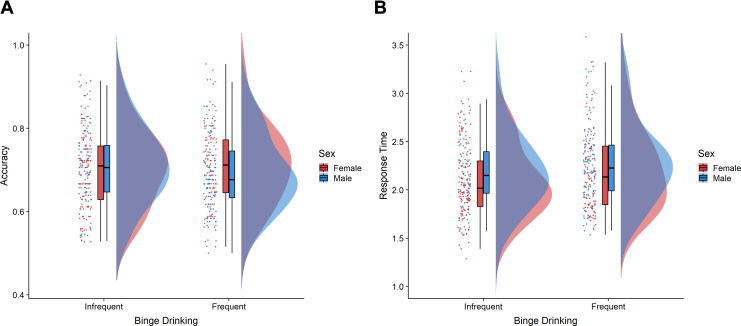
Figure 5 shows mean values for both accuracy and response times for the JLAP Test. As indicated previously, better performance is reflected in less negative values. The a priori interaction contrast (infrequent binge drinking men: +2, frequent binge drinking men: +1, infrequent binge drinking women: −2, frequent binge drinking women: −1) for accuracy precision was not significant, F(1, 200.52) = 2.50, p = .116. Inspection of the means suggests that this was because the predicted attenuation of sex differences was even more pronounced than we expected, resulting in a reversal of the advantage typically observed for men. Thus, we conducted a follow-up analysis using contrast weights informed by the observed means (infrequent binge drinking men: +2, frequent binge drinking men: −1, infrequent binge drinking women: −2, frequent binge drinking women: +1). This contrast was significant, revealing a sex difference reversal across binge drinking groups, F(1,200.52) = 7.82, p = .006, η2 = .04. While a follow-up interaction contrast should be interpreted with caution due to Type-I error inflation, the pattern of results is consistent with a priori predictions and survives a simple Bonferroni correction (.05/2).
Figure 5.
Mean values for (A) precision (lower scores mean less error) and (B) response times (seconds) by sex and binge drinking group for the Judgment of Line Angle and Position (JLAP) test.
Planned comparisons in the infrequent binge drinking group revealed that men (M = −0.24, SD = 0.81) were more accurate than women (M = −0.39, SD = 1.43), F(1,112.47) = 6.69, p = .011, d = 0.49. Men’s advantage in accuracy was not evident in the frequent binge drinking group, however (Men: M = −0.35, SD = 1.43; Women: M = −0.27, SD = 0.78), F(1,86.96) = 1.45, p = .232, d = −0.26. For response times, the interaction contrast (infrequent binge drinking men: −2, frequent binge drinking men: −1, infrequent binge drinking women: +2, frequent binge drinking women: +1) was not significant, F(1,202.91) = 1.14, p = .287.
Within the frequent binge drinking group, neither the frequency of binge episodes (> 4 drinks) nor the frequency of extreme binge episodes (> 11 drinks) was related to precision accuracy for participants of either sex, rs = .12 to .15, ps > .300.
Social-Cognitive Ability: Facial-Expression Decoding Task
Figure 6 shows mean values for both accuracy and response times (correct trials) for the facial-expression decoding task. For accuracy, the interaction contrast between sex and group (infrequent binge drinking men: −2, frequent binge drinking men: −1, infrequent binge drinking women: +2, frequent binge drinking women: +1) was not significant, F(1,408.25) = 0.10, p = .755, but it was significant for response times, F(1,415.72) = 7.22, p = .007, η2 = .02. This interaction contrast remained significant, F(1,415.39) = 5.12, p = .024, η2 < .01, even when controlling for the emotional valence of the stimuli (e.g., negative vs. neutral vs. positive). Planned comparisons in the infrequent binge drinking group indicated that women were significantly faster (M = 1.99 s, SD = 1.15) than men (M = 2.13 s, SD = 1.25) in correctly decoding facial expressions, F(1,210.88) = 5.40, p = .021, d = 0.29. Women’s advantage in speed of decoding facial expressions was not evident in the frequent binge drinking group, however (Men: M = 2.13 s, SD = 1.23; Women: M = 2.09 s, SD = 1.18), F(1,203.64) = 0.69, p = .406, d = 0.17.
Figure 6.
Mean values for both (A) accuracy and (B) response times (seconds) for the facial-expression decoding task.
Within the frequent binge drinking group, the frequency of binge drinking episodes (> 4 drinks) was unrelated to facial-expression decoding response times for women, r(98) = .16, p = .113, but women who engaged in a greater frequency of extreme binge episodes (> 11 drinks) were slower at correctly recognizing facial expressions, r(98) = .27, p = .008. For frequent binge drinking men, neither the frequency of binge episodes, r(107) = −.09, p = .340, nor the frequency of extreme binge episodes, r(107) = −.10, p = .310, was related to speed of facial-expression decoding.
As noted in the introduction, if women’s advantage in decoding facial expressions is related to female-female relational aggression, they might be particularly good at decoding the expressions of other women. The one potential exception to women’s overall advantage might be found in men’s decoding of other men’s angry facial expressions, a social signal in the context of male-male competition (Geary, 2015). Thus, we conducted follow-up analyses to test these more specific predictions—that frequent binge drinking women would be particularly disadvantaged in the processing of women’s facial expressions, and that frequent binge drinking men would show deficits in the recognition of angry male faces.
Expression decoding accuracy data did not support these predictions, as there was no significant Participant sex × Stimulus sex interaction, F(1, 1093.48) = 0.58, p = .445. However, the response time data did provide some support for these predictions. Among infrequent binge drinkers, the interaction contrast between participant sex and stimulus sex (women/female faces: −2, women/male faces: −1, men/female faces: +2, men/male faces: +1) was significant for response times, F(1, 842.62) = 7.42, p = .007, η2 = .01. This pattern indicates that women were especially fast at responding to the facial expressions of other women, relative to the facial expressions of men. Among the frequent binge drinking group, however, this interaction contrast was not significant, F(1, 946.61) = 1.71, p = .191, indicating that women’s advantage in decoding female facial expressions was not evident among frequent binge drinkers. There was no significant sex difference in response times for decoding male angry facial expressions in either the infrequent binge drinking group, F(1, 207.04) = 1.97, p = .162, or the frequent binge drinking group, F(1, 211.55) = 0.02, p = .879.
Discussion
There is now consistent evidence that men generally have better developed visuospatial abilities than women (e.g., Hyde, 2005; Jones et al., 2003; Lawton, 2010; MacDonald & Hewlett, 1999), whereas women generally have better developed social-cognitive skills than men (e.g., Hall, 1984; Merten, 2005; Thompson & Voyer, 2014). The magnitude of these sex differences varies across context, and an evolutionary perspective can situate these contextual influences in the framework of sexual selection (Darwin, 1871). Sexual selection in the context of human evolution includes visuospatial (favoring men) and social-cognitive (favoring women) sex differences that confer advantages in competition for mates or other reproductively important resources and discriminative mate choice under favorable conditions (Geary, 2021). Following Zahavi (1975) and research on condition-dependent trait expression in nonhuman species (Cotton et al., 2004; Johnstone, 1995), Geary (2015, 2019) proposed that these sex differences are condition dependent in humans, such that their development and expression is a reliable indicator of exposure to, and resistance to degradation by stressors. The current study is the first to directly test this hypothesis in humans, and to propose that recent, frequent binge drinking acts as a neurotoxic stressor disrupting cognitive abilities in sex-specific ways.
The typical advantages of men in visuospatial abilities (Voyer et al., 1995) and of women in social-cognitive abilities (Hall, 1984; Thompson & Voyer, 2014) were replicated among a group of emerging adults who never or rarely engaged in binge drinking in the past month. These sex differences were greatly attenuated or even reversed in a group of emerging adults who at least occasionally engaged in binge drinking in the recent past. Given the prevalence of binge drinking in this population—current estimates place the percentage of college student binge drinkers at 40%–50% (Croteau & Morrell, 2019; Krieger et al., 2018)—these findings suggest that sex-specific deficits among college students might be widespread. Recent data also indicate that although the prevalence of binge drinking among adolescents has declined in recent years (Chung et al., 2018), emerging and young adults are engaging in more binge drinking than in the past, reflecting a secular shift in the age of peak binge drinking (Patrick et al., 2019).
These high prevalence rates and increasing age of peak heavy episodic drinking are especially concerning in light of the current findings, given that mate competition and choice are most intense during this developmental period. During the years that coincide with elevated binge drinking rates, competition for mating-relevant resources peaks and creates a period of high risk and high reward with regard to engaging in mating effort (Hill & Chow, 2002). Indeed, binge drinking may be an attractive risk-taking behavior to emerging adults in part because it serves as a costly social signal with the potential to yield high gain in a competitive mating market (Aung et al., 2019). As would be expected of sexually selected costly signals (Zahavi, 1975), our findings highlight that binge drinking does indeed come with costs.
Under natural conditions, condition-dependent traits are vulnerable to chronic malnutrition, disease, or social conflict and appear to be more sensitive to man-made toxins than other traits (see Geary, 2015, 2019). Although heavy episodic exposure to ethyl alcohol might not be as detrimental as chronic exposure to natural stressors or many other toxins, chronic, heavy exposure to alcohol can result in short-term and sometimes longer-term but subtle deficits in memory and cognition (e.g., Goudriaan et al., 2007). Binge drinking might then reveal sex-specific vulnerabilities in visuospatial and social-cognitive abilities. Some previous studies of alcohol use have assessed similar abilities but sex differences are not always reported (Folgueira-Ares et al., 2017). When they are reported, the pattern of sex-specific deficits is mixed (Haut et al., 1989; Weissenborn & Duka, 2003). These prior studies often have been based on relatively small samples and have used standard neuropsychological measures that typically are not optimal for assessing sex-specific deficits. For instance, there are often small sex differences in spatial working memory and pattern recognition (tasks found in the Cambridge Neuropsychological Test Automated Battery; CANTAB), but sex differences on these tasks are smaller than those found for tasks used in the current study.
The difference is important because from an evolutionary perspective, sex-specific vulnerabilities generally will be more evident for traits with larger sex differences (Geary, 2017). Our results provide preliminary evidence in support of this hypothesis. Men’s advantage on both visuospatial tasks was smaller among frequent binge drinkers than among infrequent binge drinkers and non-drinkers. Moreover, there was evidence for a dose-response effect for mental rotation, whereby very high and frequent exposures to ethyl alcohol (extreme binges) were related to worse performance, but only among men. At the same time, these same men did not show exaggerated deficits in the speed of identifying emotions displayed in facial expressions. In judging line angles and position, binge drinking women were more accurate than were binge drinking men, a reversal of the standard sex difference in visuospatial abilities and of our findings for infrequent binge drinkers. We did not, however, find evidence for a dose-response effect for this measure. It is possible that men’s performance on this spatial measure is disrupted by more moderate levels of alcohol exposure with no further deficits emerging with added exposures, but this remains to be determined.
In contrast, women who recently engaged in frequent binge drinking did not show visuospatial deficits relative to women who had not engaged in binge drinking, but they were slower at identifying emotions displayed in facial expressions, especially the expressions of other women. Men often display an advantage, relative to women, in judging anger on the faces of other men (see Geary, 2015). Here, this effect did not emerge for facial-expression decoding accuracy, or for reaction time. It is possible that the task used here did not include a sufficient number of angry male faces to provide a powerful test of this effect (which was not a primary focus of this study). There also was evidence of a dose-response effect in this measure, restricted to women. That is, women who frequently engaged in extreme binges were slower at emotion detection than were other women, but these same extreme binge drinking women did not show exaggerated deficits for mental rotation. This pattern is essentially a mirror image of that observed among men who frequently engaged in extreme binges. Nevertheless, follow-up studies with larger sample sizes of binge drinkers are need to determine if there are indeed sex-specific dose-response effects for visuospatial and social-cognitive abilities.
The overall pattern of sex-specific deficits found here is consistent with the expression of condition-dependent traits in other species (Cotton et al., 2004; Johnstone, 1995), and supports the more general hypothesis that the sex differences in visuospatial and social-cognitive abilities stem from different patterns of intrasexual competition among our male and female ancestors, respectively (Geary, 2015; Geary et al., 2014). Although this study was designed based on established predictions (Geary, 2015, 2019) that provided for a priori hypothesis testing based on well-established patterns in nonhuman species, the study provides only a quasi-experimental test of those predictions. It is possible that the differences we observed across frequent and infrequent binge drinkers preceded recent drinking episodes, as suggested by modestly lower vocabulary scores among the binge drinkers. If there were broader cognitive differences across the drinking groups, however, then the frequent binge drinkers should have performed more poorly than infrequent binge drinkers on all cognitive tasks, independent of sex and not in a sex-specific manner. Moreover, because vocabulary is a good indicator of general intelligence, any binge drinking group differences on the visuospatial and social-cognitive tasks should have disappeared with statistical control of vocabulary scores, but they did not.
Different psychopathologies can also affect cognitive performance, for instance psychomotor slowing of responding in subjects with depression and anxiety (Bennabi et al., 2013; Gualtieri & Morgan, 2008). While we did not measure this in our study, and therefore could not fully control for this potential third variable, it is an interesting hypothesis to pursue in future studies. Concomitant drug use was also not measured, but can still influence cognitive performance (Davis et al., 2002; Quednow, 2017). It is currently unknown if drug use mitigates the interactive effects found here, or has an additive effect along with binge drinking frequency. Additionally, and as always, readers should interpret the results presented here with care in terms of multiple comparisons and post-hoc contrasts.
Future research would benefit from the use of a longitudinal design that would permit assessment of changes in performance on measures of purported sexually selected traits over time, as a function of changes in binge drinking frequency. Although also not an experimental design, this kind of approach would permit stronger inferences regarding the role of recent binge drinking frequency by accounting for any pre-existing differences across participants in their baseline levels of performance. Findings from such a study would further advance understanding of the extent to which exposure to this very common neurocognitive stressor specifically impairs abilities that evolutionary theory posits to be critical for sexual selection success. Despite these caveats, our results are unique and speak to the utility of using sexual selection as a means to identify and study sex-specific vulnerabilities, not just those associated with binge drinking but with exposure to myriad other potential stressors and toxins.
Simonsohn (2014) describes that for a knockout style interactive effect, sample sizes should be increased by a factor of four. Assuming a medium sized effect of d = 0.541 (see Stavro et al., 2012Tables 1 and 2), the more realistic sample size for a 2 × 2 group design on the basis of a two-group medium sized effect would be roughly 440, which is comparable to the sample size achieved in the current study.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health [F32AA025830 (PI: Hone); R01AA025451 (PI: Bartholow)].
ORCID iD: Liana S. E. Hone, MPH, PhD  https://orcid.org/0000-0002-6777-978X
https://orcid.org/0000-0002-6777-978X
References
- Andersson M. (1994). Sexual selection. Princeton University Press. [Google Scholar]
- Archer J. (2019). The reality and evolutionary significance of human psychological sex differences. Biological Reviews, 94, 1381–1415. 10.1111/brv.12507 [DOI] [PubMed] [Google Scholar]
- Asperholm M., Nagar S., Dekhtyar S., Herlitz A. (2019). The magnitude of sex differences in verbal episodic memory increases with social progress: Data from 54 countries across 40 years. PloS One, 14(4), e0214945. 10.1371/journal.pone.0214945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung T., Hughes S., Hone L. S. E., Puts D. (2019). Operational sex ratio predicts binge drinking across United States counties. Evolutionary Psychology, 17(3), 1474704919874680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennabi D., Vandel P., Papaxanthis C., Pozzo T., Haffen E. (2013). Psychomotor retardation in depression: A systematic review of diagnostic, pathophysiologic, and therapeutic implications. BioMed Research International, 1–18. 10.1155/2013/158746 [DOI] [PMC free article] [PubMed]
- Bora E., Zorlu N. (2017). Social cognition in alcohol use disorder: A meta-analysis. Addiction, 112(1), 40–48. [DOI] [PubMed] [Google Scholar]
- Bortolotti G. R., Fernie K. J., Smits J. E. (2003). Carotenoid concentration and coloration of American Kestrels (Falco sparverius) disrupted by experimental exposure to PCBs. Functional Ecology, 17, 651–657. [Google Scholar]
- Carbia C., López-Caneda E., Corral M., Cadaveira F. (2018). A systematic review of neuropsychological studies involving young binge drinkers. Neuroscience & Biobehavioral Reviews, 90, 332–349. [DOI] [PubMed] [Google Scholar]
- Card N. A., Stucky B. D., Sawalani G. M., Little T. D. (2008). Direct and indirect aggression during childhood and adolescence: A meta-analytic review of gender differences, intercorrelations, and relations to maladjustment. Child Development, 79, 1185–1229. 10.1111/j.1467-8624.2008.01184.x [DOI] [PubMed] [Google Scholar]
- Chung T., Creswell K. G., Bachrach R., Clark D. B., Martin C. S. (2018). Adolescent binge drinking. Alcohol Research: Current Reviews, 39(1), 5–15. [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. H., Isvaran K. (2007). Sex differences in ageing in natural populations of vertebrates. Proceedings of the Royal Society of London B: Biological Sciences, 274, 3097–3104. 10.1098/rspb.2007.1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaer M. L., Nelson J. D. (2002). Large visuospatial sex difference in line judgment: Possible role of attentional factors. Brain and Cognition, 49, 1–12. 10.1006/brcg.2001.1321 [DOI] [PubMed] [Google Scholar]
- Cotton S., Fowler K., Pomiankowski A. (2004). Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proceedings of the Royal Society of London B: Biological Sciences, 271, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. E., Polich J. (2010). Binge drinking effects in EEG in young adult humans. International Journal of Environmental Research and Public Health, 7, 2325–2336. 10.3390/ijerph7052325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau V., Morrell J. S. (2019). Prevalence of binge drinking episodes among male and female college students. Current Developments in Nutrition, 3(Suppl. 1), 1548. 10.1093/cdn/nzz039.P18-008-19 [DOI] [Google Scholar]
- Darwin C. (1871). The descent of man, and selection in relation to sex. John Murray. [Google Scholar]
- Davis P., Liddiard H., McMillan T. (2002). Neuropsychological deficits and opiate abuse. Drug and Alcohol Dependence, 67(1), 105–108. 10.1016/s0376-8716(02)00012-1 [DOI] [PubMed] [Google Scholar]
- Dudley R. (2000). Evolutionary origins of human alcoholism in primate frugivory. The Quarterly Review of Biology, 75(1), 3–15. [DOI] [PubMed] [Google Scholar]
- Dudley R. (2014). The drunken monkey: Why we drink and abuse alcohol. University of California Press. [Google Scholar]
- Ekstrom R. B., French J. W., Harman H. H. (1976). Manual for kit of factor-referenced cognitive tests. Educational Testing Service. [Google Scholar]
- Fama R. (2019). Introduction to the special section on alcohol: Review of cognitive, emotional, and neural deficits and recovery with sustained abstinence and treatment. Neuropsychology, 33(6), 757. [DOI] [PubMed] [Google Scholar]
- Folgueira-Ares R., Cadaveira F., Rodríguez Holguín S., López-Caneda E., Crego A., Pazo-Álvarez P. (2017). Electrophysiological anomalies in face–name memory encoding in young binge drinkers. Frontiers in Psychiatry, 8, 216. 10.3389/fpsyt.2017.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary D. C. (2015). Evolution of vulnerability: Implications for sex differences in health anddevelopment. Elsevier Academic Press. [Google Scholar]
- Geary D. C. (2017). Evolution of human sex-specific cognitive vulnerabilities. The Quarterly Review of Biology, 92, 361–410. 10.1086/694934 [DOI] [Google Scholar]
- Geary D. C. (2019). Evolutionary perspective on sex differences in the expression of neurological diseases. Progress in Neurobiology, 176, 33–53. 10.1016/j.pneurobio.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Geary D. C. (2021). Male, female: The evolution and human sex differences (3rd ed.). American Psychological Association. [Google Scholar]
- Geary D. C., Saults S. J., Liu F., Hoard M. K. (2000). Sex differences in spatial cognition, computational fluency, and arithmetic reasoning. Journal of Experimental Child Psychology, 77(4), 337–353. 10.1006/jecp.2000.2594 [DOI] [PubMed] [Google Scholar]
- Geary D. C., Winegard B., Winegard B. (2014). Reflections on the evolution of human sex differences: Social selection and the evolution of competition among women. In Weekes-Shackelford V. A., Shackelford T. K. (Eds.), Evolutionary perspectives on human sexual psychology and behavior (pp. 395–414). Springer. [Google Scholar]
- Goudriaan A. E., Grekin E. R., Sher K. J. (2007). Decision making and binge drinking: A longitudinal study. Alcoholism: Clinical and Experimental Research, 31(6), 928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri C. T., Morgan D. W. (2008). The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder. The Journal of Clinical Psychiatry, 69(7), 1122–1130. 10.4088/jcp.v69n0712 [DOI] [PubMed] [Google Scholar]
- Hall J. A. (1984). Nonverbal sex differences: Communication accuracy and expressive style. The Johns Hopkins University Press. [Google Scholar]
- Hall J. A., Matsumoto D. (2004). Gender differences in judgments of multiple emotions from facial expressions. Emotion, 4(2), 201–206. 10.1037/1528-3542.4.2.201 [DOI] [PubMed] [Google Scholar]
- Hartley D. E., Elsabagh S., File S. E. (2004). Binge drinking and sex: effects on mood and cognitive function in healthy young volunteers. Pharmacology Biochemistry and Behavior, 78, 611–619. 10.1016/j.pbb.2004.04.027 [DOI] [PubMed] [Google Scholar]
- Haut J. S., Beckwith B. E., Petros T. V., Russell S. (1989). Gender differences in retrieval from long-term memory following acute intoxication with ethanol. Physiology & Behavior, 45, 1161–1165. 10.1016/0031-9384(89)90103-0 [DOI] [PubMed] [Google Scholar]
- Hill E. M., Chow K. (2002). Life-history theory and risky drinking. Addiction, 97(4), 401–413. [DOI] [PubMed] [Google Scholar]
- Hindmarch I., Kerr J. S., Sherwood N. (1991). The effects of alcohol and other drugs on psychomotor performance and cognitive function. Alcohol & Alcoholism, 26(1), 71–79. 10.1016/j.marpolbul.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Howland J., Rohsenow D. J., Greece J. A., Littlefield C. A., Almeida A., Heeren T., Winter M., Bliss C. A., Hunt S., Hermos J. (2010). The effects of binge drinking on college students’ next-day academic test-taking performance and mood state. Addiction, 105(4), 655–665. 10.1111/j.1360-0443.2009.02880.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde J. S. (2005). The gender similarities hypothesis. American Psychologist, 60, 581–592. https://doi.org/1037/0003-066X.60.6.581 [DOI] [PubMed] [Google Scholar]
- Jacobus J., Tapert S. F. (2013). Neurotoxic effects of alcohol in adolescence. Annual Review of Clinical Psychology, 9(1), 703–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowiak W., Sudakov M., Wilreker B. C. (2005). Co-wife conflict and co-operation. Ethnology, 44, 81–98. 10.2307/3773961 [DOI] [Google Scholar]
- Jašarević E., Sieli P. T., Twellman E. E., Welsh T. H., Jr, Schachtman T. R., Roberts R. M., Geary D. C., Rosenfeld C. S. (2011). Disruption of adult expression of sexually selected traits by early exposure to Bisphenol A. Proceedings of the National Academy of Sciences of the United States of America, 108, 11715–11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A. R. (1998). The g factor: The science of mental ability. Praeger. [Google Scholar]
- Ji T., Wu J. J., He Q. Q., Xu J. J., Mace R., Tao Y. (2013). Reproductive competition between females in the matrilineal Mosuo of southwestern China. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 368, 20130081. 10.1098/rstb.2013.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone R. A. (1995). Sexual selection, honest advertisement and the handicap principle: Reviewing the evidence. Biological Reviews, 70, 1–65. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Braithwaite V. A., Healy S. D. (2003). The evolution of sex differences in spatial ability. Behavioral Neuroscience., 117, 304–411. [DOI] [PubMed] [Google Scholar]
- Krieger H., Young C. M., Anthenien A. M., Neighbors C. (2018). The epidemiology of binge drinking among college-age individuals in the United States. Alcohol Research: Current Reviews, 39(1), 23–30. [PMC free article] [PubMed] [Google Scholar]
- Lannoy S., Billieux J., Dormal V., Maurage P. (2019). Behavioral and cerebral impairments associated with binge drinking in youth: A critical review. Psychologica Belgica, 59(1), 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoy S., Dormal V., Brion M., Gaudelus B., Billieux J., Maurage P. (2018). Affective impairments in binge drinking: Investigation through emotional facial expression decoding. Comprehensive Psychiatry, 83, 59–63. 10.1016/j.comppsych.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Lawton C. A. (2010). Gender, spatial abilities, and wayfinding. In Chrisler J. C., McCreary D. R. (Eds.), Handbook of gender research in psychology (pp. 317–341). Springer. [Google Scholar]
- Leigh S. R. (1995). Socioecology and the ontogeny of sexual size dimorphism in anthropoid primates. American Journal of Physical Anthropology, 97, 339–356. [DOI] [PubMed] [Google Scholar]
- Loyau A., Saint Jalme M., Cagniant C., Sorci G. (2005). Multiple sexual advertisements honestly reflect health status in peacocks (Pavo cristatus). Behavioral Ecology and Sociobiology, 58, 552–557. 10.1007/s00265-005-0958-y [DOI] [Google Scholar]
- MacDonald D. H., Hewlett B. S. (1999). Reproductive interests and forager mobility. Current Anthropology, 40, 501–523. 10.1086/200047 [DOI] [Google Scholar]
- Martins J. S., Bartholow B. D., Cooper M. L., Von Gunten C. D., Wood P. K. (2018). Associations between executive functioning, affect-regulation drinking motives, and alcohol use and problems. Psychology of Addictive Behaviors, 32(1), 16–28. 10.1037/adb0000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschek H., Kliegl R., Vasishth S., Baayen H., Bates D. (2017). Balancing type I error and power in linear mixed models. Journal of Memory and Language, 94, 305–315. 10.1016/j.jml.2017.01.001 [DOI] [Google Scholar]
- Maurage P., Joassin F., Speth A., Modave J., Philippot P., Campanella S. (2012). Cerebral effects of binge drinking: Respective influences of global alcohol intake and consumption pattern. Clinical Neurophysiology, 123(5), 892–901. 10.1016/j.clinph.2011.09.018 [DOI] [PubMed] [Google Scholar]
- McClure E. B. (2000). A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychological Bulletin, 126(3), 424–453. 10.1037/0033-2909.126.3.424 [DOI] [PubMed] [Google Scholar]
- Merten J. (2005). Culture, gender and the recognition of the basic emotions. Psychologia, 48(4), 306–316. 10.2117/psysoc.2005.306 [DOI] [Google Scholar]
- National Institutes on Alcohol Abuse and Alcoholism. (2004). NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsletter, Winter(3), 3. [Google Scholar]
- Nesse R. M. (2002). Evolution and addiction. Addiction, 97, 470–474. [DOI] [PubMed] [Google Scholar]
- Nixon S. J., Prather R., Lewis B. (2014). Sex differences in alcohol-related neurobehavioral consequences. In Handbook of clinical neurology (1st ed., Vol. 125). Elsevier B.V. 10.1016/B978-0-444-62619-6.00016-1 [DOI] [PubMed] [Google Scholar]
- Obernier J. A., White A. M., Swartzwelder H. S., Crews F. T. (2002). Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacology Biochemistry and Behavior, 72(3), 521–532. 10.1016/S0091-3057(02)00715-3 [DOI] [PubMed] [Google Scholar]
- Parsons O. A., Nixon S. J. (1993). Neurobehavioral sequelae of alcoholism. Neurologic Clinics, 11, 205–218. [PubMed] [Google Scholar]
- Patrick M. E., Terry‐McElrath Y. M., Lanza S. T., Jager J., Schulenberg J. E., O’Malley P. M. (2019). Shifting age of peak binge drinking prevalence: Historical changes in normative trajectories among young adults aged 18 to 30. Alcoholism: Clinical and Experimental Research, 43(2), 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins J. M., Subramanian S. V., Davey Smith G., Özaltin E. (2016). Adult height, nutrition, and population health. Nutrition Reviews, 74, 149–165. 10.1093/nutrit/nuv105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M., Laeng B., Latham K., Jackson M., Zaiyouna R., Richardson C. (1995). A redrawn Vandenberg and Kuse mental rotations test-different versions and factors that affect performance. Brain and Cognition, 28, 39–58. 10.1006/brcg.1995.1032 [DOI] [PubMed] [Google Scholar]
- Psychology Software Tools. (2016). E-Prime 3.0. Psychology Software Tools. [Google Scholar]
- Quednow B. B. (2017). Social cognition and interaction in stimulant use disorders. Current Opinion in Behavioral Sciences, 13, 55–62. 10.1016/j.cobeha.2016.10.001 [DOI] [Google Scholar]
- Rosnow R. L., Rosenthal R. (1995). “Some things you learn aren’t so”: Cohen’s Paradox, Asch’s paradigm, and the interpretation of interaction. Psychological Science, 6(1), 3–9. 10.1111/j.1467-9280.1995.tb00297.x [DOI] [Google Scholar]
- Rotter N. G., Rotter G. S. (1988). Sex differences in the encoding and decoding of negative facial emotions. Journal of Nonverbal Behavior, 12, 139–148. [Google Scholar]
- Rourke S. B., Grant I. (2009). The Neurobehavioral correlates of alcoholism. In Grant I., Adams K. (Eds.), Neuropsychological assessment of neuropsychiatric and neuromedical disorders (pp. 398–454). Oxford University Press. [Google Scholar]
- Royston P., Altman D. G., Sauerbrei W. (2005). Dichotomizing continuous predictors in multiple regression: A bad idea. Statistics in Medicine, 25(1), 127–141. 10.1002/sim.2331 [DOI] [PubMed] [Google Scholar]
- Simonsohn U. (2014, March12). No-way interactions. Data Colada. 10.15200/winn.142559.90552 [DOI]
- Smuts B. B. (1987). Gender, aggression, and influence. In Smuts B. B., Cheney D. L., Seyfarth R. M., Wrangham R. W., Struhsaker T. T. (Eds.), Primate societies (pp. 400–412). The University of Chicago Press. [Google Scholar]
- Squeglia L. M., Spadoni A. D., Infante M. A., Myers M. G., Tapert S. F. (2009). Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors, 23(4), 715–722. 10.1037/a0016516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K., Pelletier J., Potvin S. (2012). Widespread and sustained cognitive deficits in alcoholism: A meta-analysis. Addiction Biology, 18(2), 203–213. 10.1111/j.1369-1600.2011.00418 [DOI] [PubMed] [Google Scholar]
- Stockley P., Campbell A. (2013). Female competition and aggression: Interdisciplinary perspectives. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 368, 20130073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E. V., Rosenbloom M. J., Pfefferbaum A. (2000). Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism: Clinical and Experimental Research, 24, 611–621. doi: 10.1111/j.1530-0277.2000.tb02032.x [PubMed] [Google Scholar]
- Sullivan E. V., Fama R., Rosenbloom M. J., Pfefferbaum A. (2002). A profile of neuropsychological deficits in alcoholic women. Neuropsychology, 16, 74–83. doi: 10.1037/0894-4105.16.1.74 [DOI] [PubMed] [Google Scholar]
- Sullivan E. V. (2017). Contributions to understanding the neuropsychology of alcoholism: An INS legacy. Journal of the International Neuropsychological Society, 23(9-10), 843–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnik B. G., Fidell L. S. (2012). Using multivariate statistics (6th ed.). Pearson. [Google Scholar]
- Tanner J. M. (1990). Foetus into man: Physical growth from conception to maturity. Harvard University Press. [Google Scholar]
- Thompson A. E., Voyer D. (2014). Sex differences in the ability to recognise non-verbal displays of emotion: A meta-analysis. Cognition and Emotion, 28, 1164–1195. 10.1080/02699931.2013.875889 [DOI] [PubMed] [Google Scholar]
- Townshend J. M., Duka T. (2005). Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcoholism: Clinical and Experimental Research, 29(3), 317–325. [DOI] [PubMed] [Google Scholar]
- Valmas M. M., Mosher Ruiz S., Gansler D. A., Sawyer K. S., Oscar‐Berman M. (2014). Social cognition deficits and associations with drinking history in alcoholic men and women. Alcoholism: Clinical and Experimental Research, 38(12), 2998–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek Y., Dubas J. S. (2008). Age and gender differences in decoding basic and non-basic facial expressions in late childhood and early adolescence. Journal of Nonverbal Behavior, 32, 37–52. 10.1007/s10919-007-0040-8 [DOI] [Google Scholar]
- Vashro L., Cashdan E. (2015). Spatial cognition, mobility, and reproductive success in northwestern Namibia. Evolution and Human Behavior, 36, 123–129. 10.1016/j.evolhumbehav.2014.09.009 [DOI] [Google Scholar]
- Voyer D., Voyer S. D., Saint-Aubin J. (2017). Sex differences in visual-spatial working memory: A meta-analysis. Psychonomic Bulletin & Review, 24, 307–334. 10.3758/s13423-016-1085-7 [DOI] [PubMed] [Google Scholar]
- Voyer D., Voyer S., Bryden M. P. (1995). Magnitude of sex differences in spatial abilities: A meta-analysis and consideration and consideration of critical variables. Psychological Bulletin, 117, 250–270. 10.1037/0033-2909.117.2.250 [DOI] [PubMed] [Google Scholar]
- Weissenborn R., Duka T. (2003). Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. Psychopharmacology, 165, 306–312. 10.1007/s00213-002-1281-1 [DOI] [PubMed] [Google Scholar]
- Wood W., Eagly A. H. (2002). A Cross-cultural analysis of the behavior of women and men: Implications for the origins of sex differences. Psychological Bulletin, 128, 699–727. 10.1037/0033-2909.128.5.699 [DOI] [PubMed] [Google Scholar]
- Zahavi A. (1975). Mate selection—A selection for a handicap. Journal of Theoretical Biology, 53, 205–214. https://doi.org/https://10.1016/0022-5193(75)90111-3 [DOI] [PubMed] [Google Scholar]