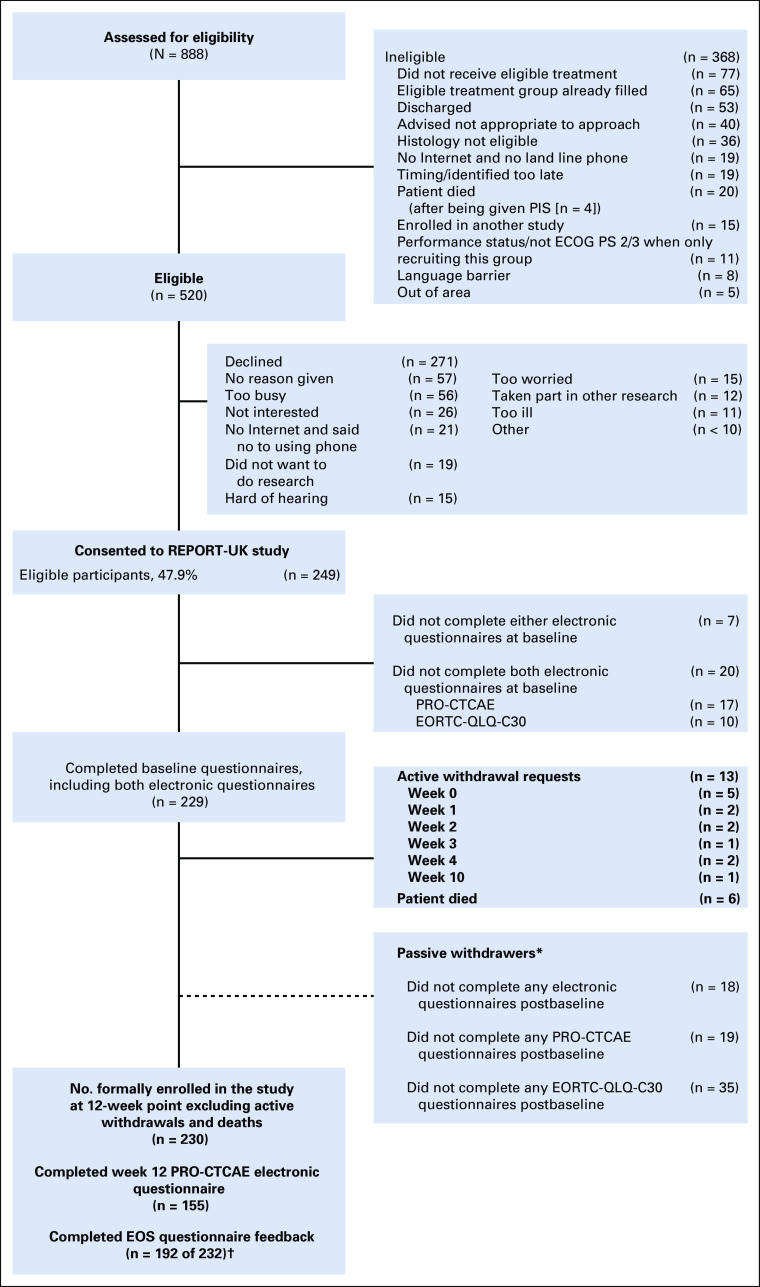
FIG 1.
Study recruitment CONSORT diagram. (*) Passive withdrawers are those who did not complete the online questionnaires postbaseline but who did not formally request to be withdrawn. (NOTE. These were included as expected in the compliance calculations.) (†) Two patients who withdrew were sent and completed the (end-of-study (EOS) questionnaire as they had used the systems for some time. ECOG PS, Eastern Cooperative Oncology Group performance status; EORTC-QLQ-C30, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire C30; PIS, Patient Information Sheet; PRO-CTCAE, Patient Reported Outcomes version of the Common Terminology Criteria for Adverse Events.