FIG 2.
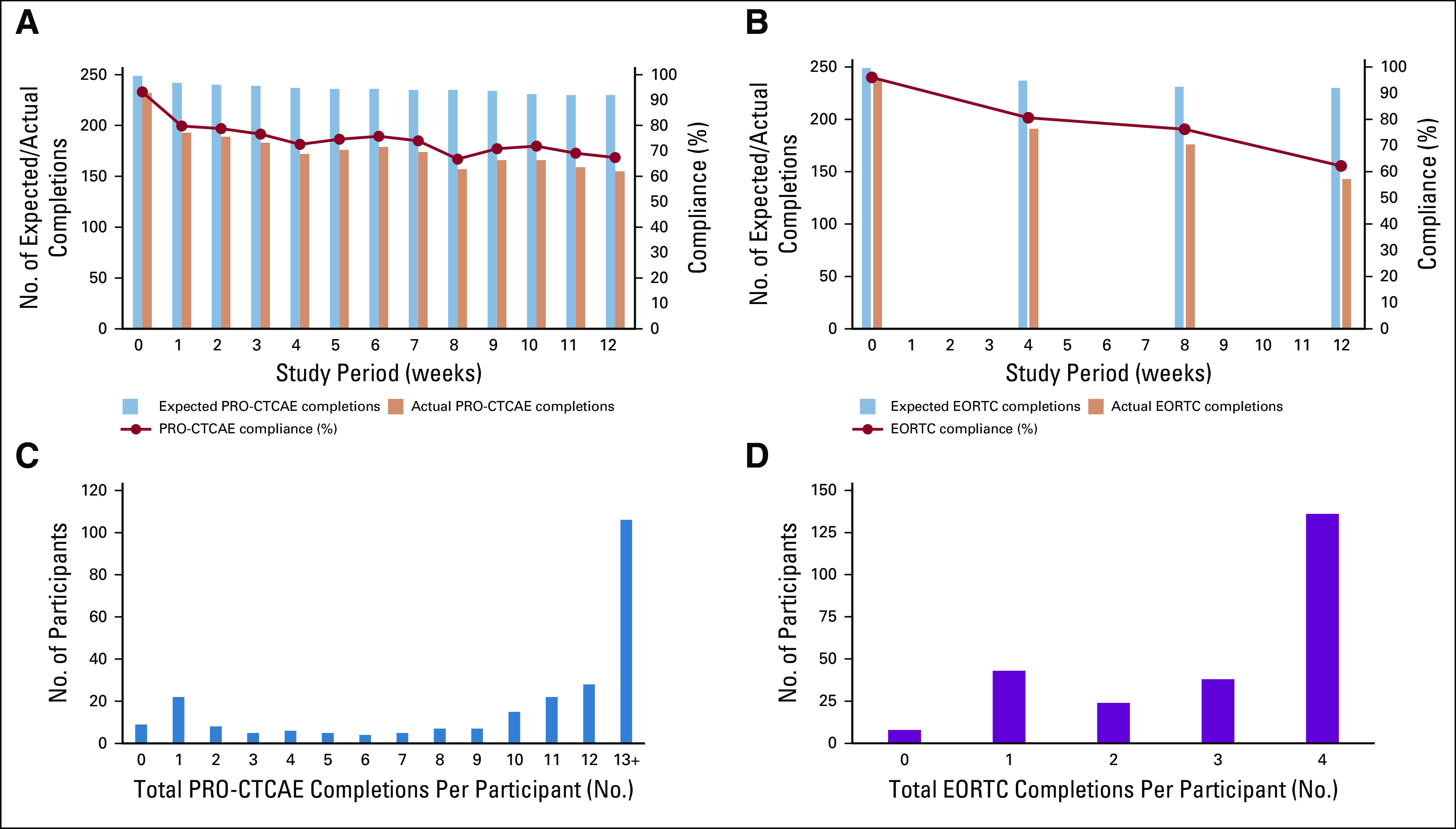
Compliance and number of completions per participant for PRO-CTCAE (Patient Reported Outcomes version of the Common Terminology Criteria for Adverse Events) and EORTC (European Organization for Research and Treatment of Cancer QLQ-C30) questionnaires. (A) PRO-CTCAE weekly compliance across the 12-week study period. (B) EORTC compliance every 4 weeks across the 12-week study period. (C) Total number of PRO-CTCAE completions per participant. (D) Total number of EORTC completions per participant.
