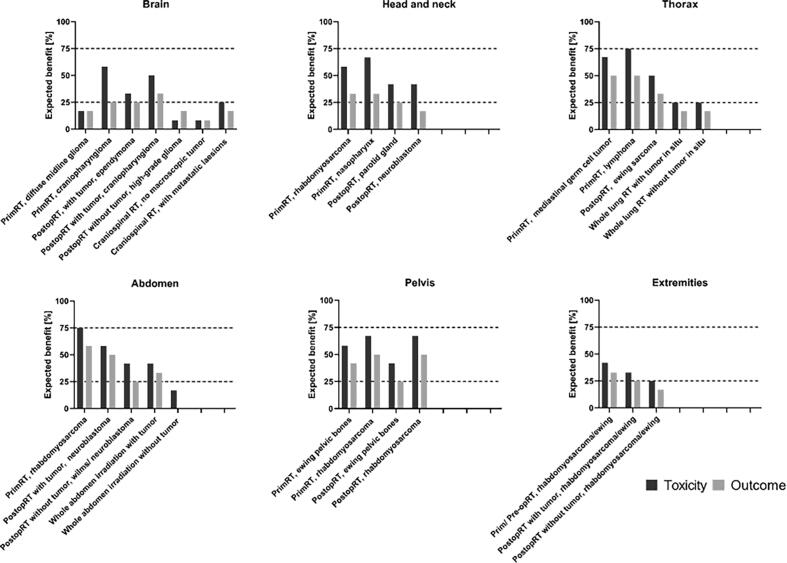
Fig. 1.
Illustration of expected benefit from MRgRT for primary pediatric tumor sites. Percentage of responders (N = 12) expecting a benefit of toxicity reduction or clinical outcome improvement by the use of MRgRT compared to a CBCT-guided photon or proton therapy for different areas in the body and common pediatric tumor scenarios. A percentage of ≤25%, 25–75% and ≥75% were considered as ‘no’, ‘possible’, and ‘expected clinical benefit’, respectively. PrimRT = primary radiotherapy, PostopRT = post operative radiotherapy.