Figure 1.
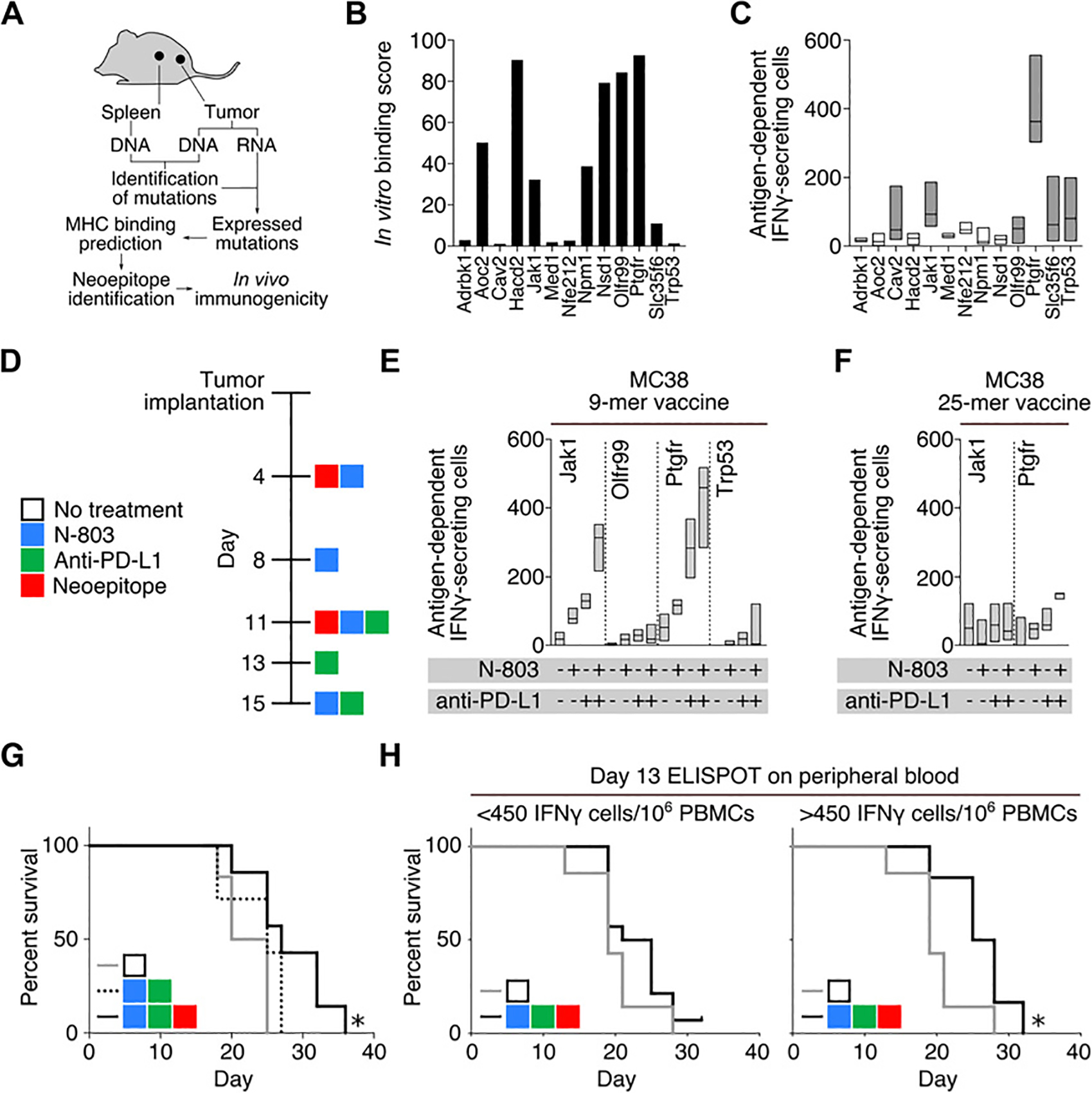
Identification of immunogenic neoepitopes. A, Workflow for neoepitope discovery in subcutaneously implanted MC38 tumors. B, In vitro binding score of MC38 neoepitopes. C, IFNγ ELISPOT analysis of naïve mice vaccinated twice with pools of 9-mer neoepitopes. Splenocytes were harvested 1 week following the second vaccination. Shaded bars represent peptides that induced robust immune responses in repeated experiments (n = 4). D, Treatment schedule. Day 18 IFNγ ELISPOT analysis of mice vaccinated with four 9-mer (n = 3; E) or two 25-mer neoepitopes (n = 3; F), alone or in combination with N-803 and/or anti–PD-L1. G, Survival curves of tumor-bearing mice treated with N-803, anti–PD-L1, and four 9-mer neoepitope peptides (black line), N-803, anti–PD-L1, and PBS (dashed line), or no treatment (gray line) (n = 6–7). H, Survival curves of mice treated with 9-mer neoepitope vaccine, N-803, and anti–PD-L1 stratified by antigen-specific IFNγ-secreting cells per 106 cells in the peripheral blood on day 13, *, P < 0.05, (n = 7–9). Data are representative of 1 to 2 independent experiments.
