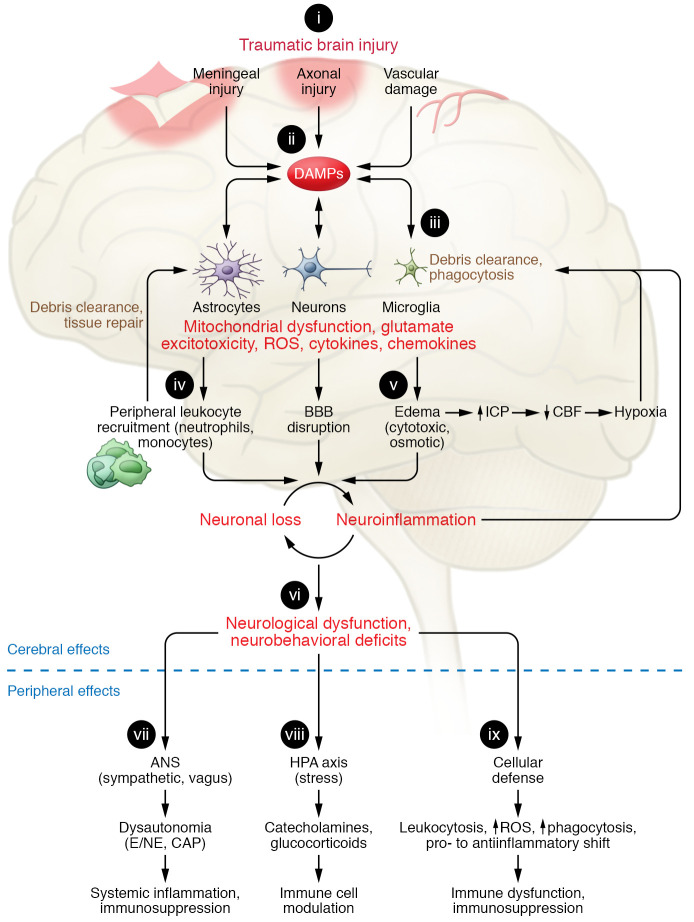
Figure 1. Innate immune responses in brain and periphery following traumatic brain injury.
(i) Depending on the severity of traumatic brain injury (TBI), the primary mechanical injury consists of meningeal contusion, axonal shearing, and cerebrovascular injury that leads to meningeal and neuronal cell death and activation of microglia and astrocytes. (ii) Neuronal injury and glial activation generate chemokines, cytokines, and reactive oxygen species (ROS) and release of damage-associated molecular patterns (DAMPs), eliciting an inflammatory response. (iii) When exposed to DAMPs, phagocytic microglia clear debris and produce neurotrophic factors. (iv) Chronic stimulation of these pathways induces secondary injury via recruitment of leukocytes, which initially facilitate clearance of tissue debris but then contribute to progression of inflammation and blood-brain barrier (BBB) breakdown. (v) The ensuing cytotoxic edema and impaired BBB function increase intracranial pressure (ICP) and lead to reduced cerebral blood flow (CBF), amplifying hypoxia to disrupt energy supply in the brain. This causes further neuronal loss and a feed-forward cycle of neuroinflammation and neurodegeneration. (vi) These progressive pathological changes lead to neurological dysfunction and deficits in motor, cognitive, and affective function. TBI also alters the autonomic nervous system (ANS), which is hard-wired to monitor and modulate DAMPs, thereby producing extracerebral and peripheral innate immune responses. (vii) Sympathetic ANS activation results in peripheral release of catecholamines (epinephrine/norepinephrine; E/NE), which suppress systemic immune responses. The vagus stimulates splenic T lymphocytes and inhibits proinflammatory responses of macrophages, via the cholinergic antiinflammatory pathway (CAP) that dampens systemic inflammation. (viii) The release of catecholamines and glucocorticoids via the hypothalamic-pituitary-adrenal (HPA) axis modulates systemic immune cell function after TBI. (ix) TBI can also disrupt systemic cellular defense mechanisms.