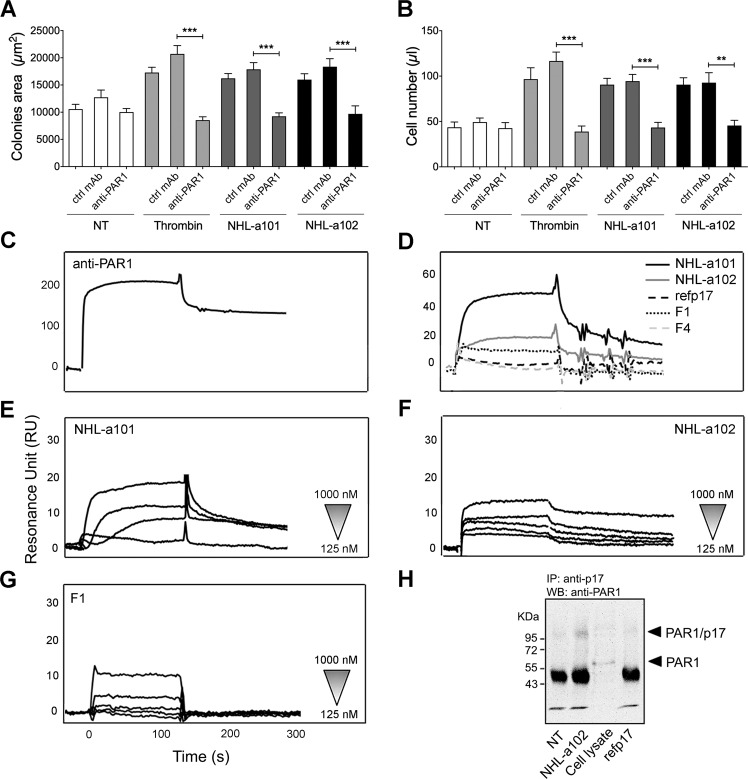
Fig. 8. The vp17s-induced B-cell clonogenic activity is mediated by their interaction with PAR1.
A Raji cells were cultured for 8 days in the presence or absence of NHL-a101 or NHL-a102 (10 ng/ml) or thrombin (2 U/ml) and neutralizing mAb ATAP2 to PAR1 (anti-PAR1) or isotype control mAb (Ctrl mAb; 1 μg/ml). The colony area of Raji was measured (15 colonies/condition) by using Leica Qwin image analysis software. B The same number of colonies (15 colonies/condition) was aseptically harvested from 96-well plates and stained with propidium iodide (PI) to detect PI-viable cells by flow cytometry. Absolute cell counts were obtained by the counting function of the MACSQuant® Analyzer. Bars represent the means ± SD of three independent experiments. The statistical significance between control and treated cultures was calculated using one-way ANOVA and the Bonferroni’s post-test was used to compare data. NT, not treated. **P < 0.01; ***P < 0.001. C–G SPR analysis of the interaction of vp17s and F1 peptide with PAR1. C Blank subtracted sensorgram showing the binding of anti-PAR1 mAb (ATAP2). D Representative blank subtracted sensorgrams of refp17, NHL-a101, NHL-a102, F1, and F4 binding. E, F, G Overlay of blank subtracted sensorgrams, resulting from the injection of increasing concentration (from 125 to 1000 nM) of NHL-a101 (E), NHL-a102 (F) and F1 (G), used to determinate the kinetic parameter of interaction. RU, resonance unit. H Co-immunoprecipitation of p17/PAR1 complexes. Raji cells were incubate or not for 15 min at room temperature with 1.5 μg of vp17 NHL-a102 or refp17. After chemical cross-linking, PAR1 was immunoprecipitated from the lysates with an anti-p17 antibody (MBS-34). The immunoprecipitates were detected by western blotting using a mAb to PAR1 (ATAP2). One representative experiment of three with similar results is shown. NT, not treated.