Abstract
Antimicrobial resistance is mostly studied by means of phenotypic growth inhibition determinations, in combination with PCR confirmations or further characterization by means of whole genome sequencing (WGS). However, the actual proteins that cause resistance such as enzymes and a lack of porins cannot be detected by these methods. Improvements in liquid chromatography (LC) and mass spectrometry (MS) enabled easier and more comprehensive proteome analysis. In the current study, susceptibility testing, WGS and MS are combined into a multi-omics approach to analyze resistance against frequently used antibiotics within the beta-lactam, aminoglycoside and fluoroquinolone group in E. coli and K. pneumoniae. Our aim was to study which currently known mechanisms of resistance can be detected at the protein level using liquid chromatography–mass spectrometry (LC–MS/MS) and to assess whether these could explain beta-lactam, aminoglycoside, and fluoroquinolone resistance in the studied isolates. Furthermore, we aimed to identify significant protein to resistance correlations which have not yet been described before and to correlate the abundance of different porins in relation to resistance to different classes of antibiotics. Whole genome sequencing, high-resolution LC–MS/MS and antimicrobial susceptibility testing by broth microdilution were performed for 187 clinical E. coli and K. pneumoniae isolates. Resistance genes and proteins were identified using the Comprehensive Antibiotic Resistance Database (CARD). All proteins were annotated using the NCBI RefSeq database and Prokka. Proteins of small spectrum beta-lactamases, extended spectrum beta-lactamases, AmpC beta-lactamases, carbapenemases, and proteins of 16S ribosomal RNA methyltransferases and aminoglycoside acetyltransferases can be detected in E. coli and K. pneumoniae by LC–MS/MS. The detected mechanisms matched with the phenotype in the majority of isolates. Differences in the abundance and the primary structure of other proteins such as porins also correlated with resistance. LC–MS/MS is a different and complementary method which can be used to characterize antimicrobial resistance in detail as not only the primary resistance causing mechanisms are detected, but also secondary enhancing resistance mechanisms.
Subject terms: Antimicrobial resistance, Proteomics
Introduction
Antimicrobial resistance (AMR) is widely recognized as a serious threat to global public health1. Among drug resistant pathogens, antibiotic resistant Escherichia coli and Klebsiella pneumoniae are particularly worrisome. Both species belong to the family of Enterobacterales and are clinically relevant as they frequently cause various infections in patients of all ages. Infections caused by these bacteria include urinary tract infection, cholangitis and sepsis2,3. Both organisms have become increasingly resistant to several antibiotics. A recent study estimated that third-generation cephalosporin-resistant E. coli and K. pneumoniae were responsible for approximately 300,000 and 70,000 infections in the European Economic Area in 20154. Furthermore, the WHO considers the development of new antibiotics critical to combat carbapenem resistant and extended-spectrum beta-lactamase (ESBL) producing Enterobacterales5.
To better diagnose and treat antibiotic resistant micro-organisms, a thorough understanding of AMR-mechanisms is paramount. In the last three decades, our understanding of AMR-mechanisms has increased significantly by unravelling their genome using DNA sequencing. Currently, whole genome sequencing (WGS) has advanced to a point where complete bacterial genomes can be sequenced within hours. The genetic bases of many AMR-mechanisms are known, and both resistance and susceptibility to some antibiotics can be predicted for important pathogens using WGS6–8.
However, the detection of genes encoding resistance mechanisms does not provide information on the subsequent transcription and translation processes resulting in different protein quantities and enzymatic activity, and it does also not provide information on the interaction between different resistance mechanisms, e.g., a decrease in porins combined with increased beta-lactamase production9–11. One of the ways to detect and assess the abundance of these mechanisms is by analysis of the bacterial proteome. Similar to the achievements made for WGS, liquid chromatography combined with mass spectrometry (LC–MS) has advanced to a level in which proteomes can be comprehensively characterized within a few hours using bottom-up shotgun proteomics12. Both Chang et al. and Trip et al. applied this discovery-based approach to detect beta-lactamases in several Acinetobacter baumannii isolates13,14. In addition to discovery-based approaches which are mainly used in a research setting, targeted protein detection methods are being developed that show potential for diagnostic testing9,15,16. Besides being highly accurate, these methods offer a shorter turnaround time than the currently applied phenotypical susceptibility testing techniques that require overnight incubation which results in a delay in reporting time17.
In the current study, we analyzed which antimicrobial resistance genes were detected at the protein level using LC–MS/MS without applying antibiotic pressure. The detected genes and their respective proteins were subsequently matched with the susceptibility results obtained for beta-lactam, aminoglycoside, and fluoroquinolone antibiotics. Furthermore, we studied the relationship between the abundance of different porins and whether this correlated to resistance against different classes of antibiotics. Finally, we identified protein to resistance correlations that have not yet been described before. For these objectives, data-dependent acquisition (DDA) LC–MS/MS, WGS and antimicrobial susceptibility testing (AST) were performed for a selected set of 187 E. coli and K. pneumoniae isolates containing various antibiotic resistance mechanisms.
Results
Sample characteristics
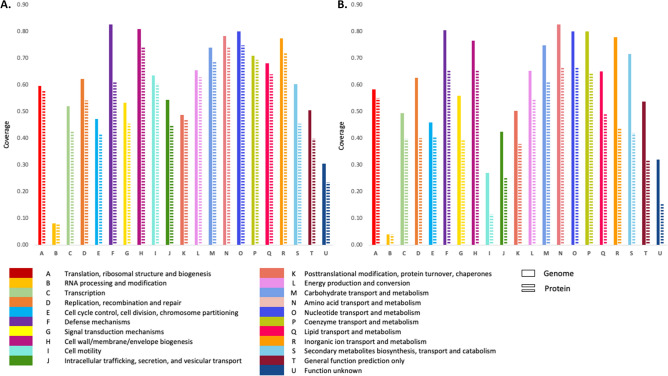
In the current study, 187 E. coli and K pneumoniae isolates were included displaying a wide variety of beta-lactam, aminoglycoside and quinolone resistance mechanisms. We included isolates containing small spectrum and extended spectrum beta-lactamases, carbapenemases and isolates containing different aminoglycoside modifying enzymes as well as 16S rRNA methyltransferases. Before analyzing resistance mechanisms, we first determined to which extent high-resolution mass spectrometry was capable of detecting and characterizing the proteome of the E. coli and K. pneumoniae isolates in comparison to the information obtained by WGS. For this purpose, clusters of orthologous groups of proteins (COG) analysis was performed18. In Fig. 1, the correlation of all proteins and genes that were detected at least once are shown for the major functional families. Proteins were detected in each functional family of which genes were detected with none of the families showing a particular low protein to gene ratio. On average 2174 (± 56) proteins were detected per E. coli isolate and 2302 (± 69) proteins per K. pneumonia isolate. This corresponded with an average predicted protein coverage by LC–MS/MS of 0.46 in E. coli (4747 ± 230 predicted proteins) and 0.44 in K. pneumonia (5199 ± 188 predicted proteins).
Figure 1.
Clusters of orthologous groups (COG) analysis of all genes and all proteins which were detected at least once in the E. coli isolates (A) and in the K. pneumoniae isolates (B). X-axis: The major functional COG families. Y-axis: The proportion of genes and proteins that were detected in both species compared to the COG database. Genes are represented by solid bars and proteins are represented by interrupted bars.
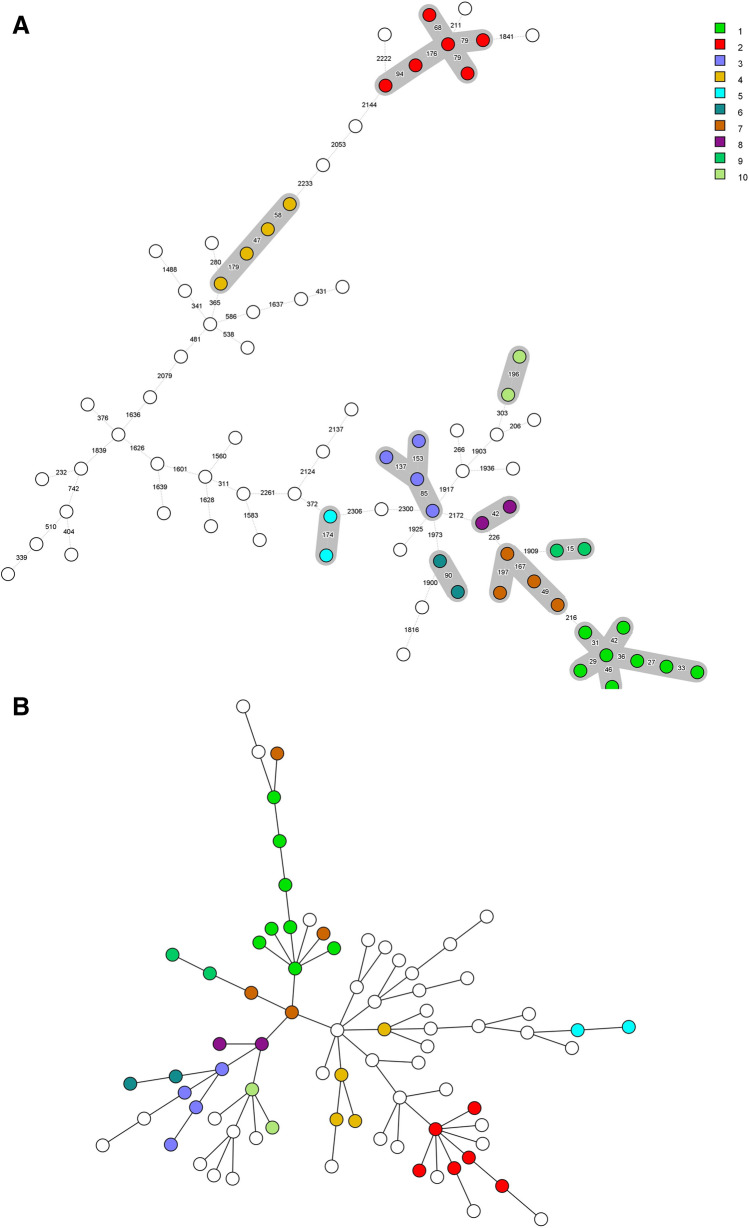
To demonstrate the heterogeneity of the selected isolates harboring different resistance mechanisms, clustering based on core genome multilocus sequence typing (cgMLST) and clustering based on protein intensity were compared. For E. coli, both the cgMLST as well as clustering based on protein intensity showed a diverse set of isolates with some clustering but all isolates differed by at least 15 alleles. The K. pneumoniae isolates were also diverse but several clusters of isolates were present. In general, both cgMLST and protein intensity analysis showed similar clustering of isolates (Fig. 2).
Figure 2.
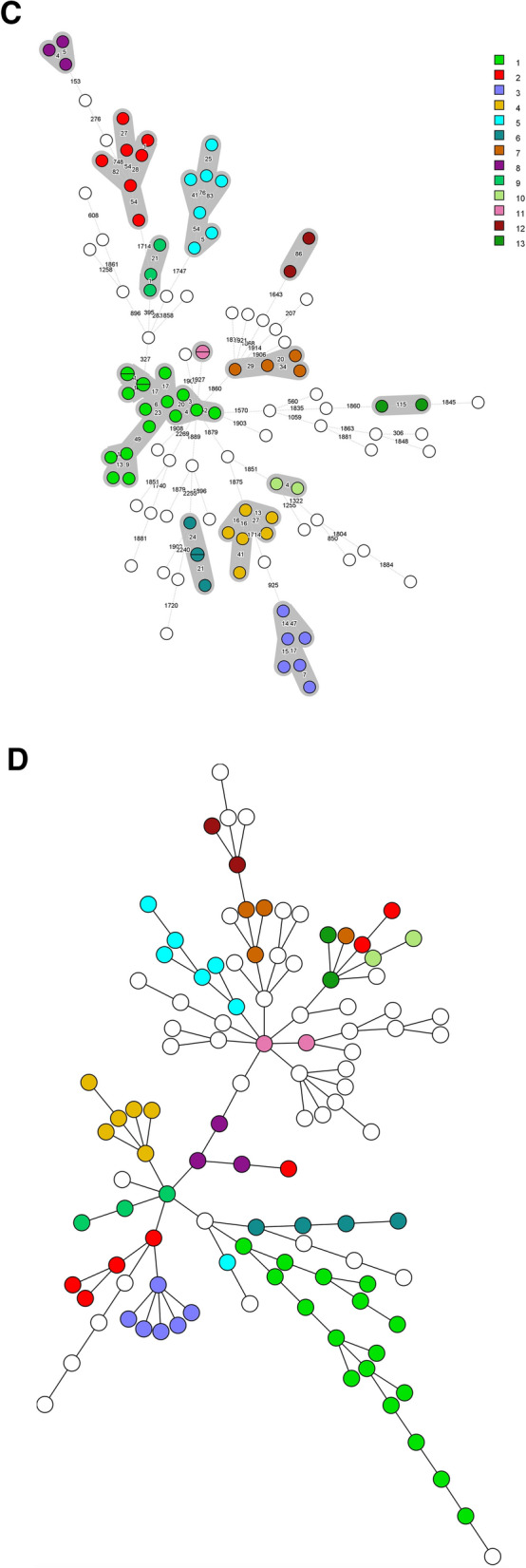
A comparison of sample clustering based on core genome multilocus sequence typing (cgMLST) and clustering based on protein intensity. Each circle represents one isolate. (A, B) Display the clustering obtained by cgMLST and protein intensity analysis for the 78 E. coli isolates. (C, D) Display the clustering obtained by cgMLST and protein intensity analysis for the 109 K. pneumoniae isolates. Each cluster was indicated with a color and numbered. The same isolates were colored in both the cgMLST and protein intensity analysis.
Beta-lactam resistance
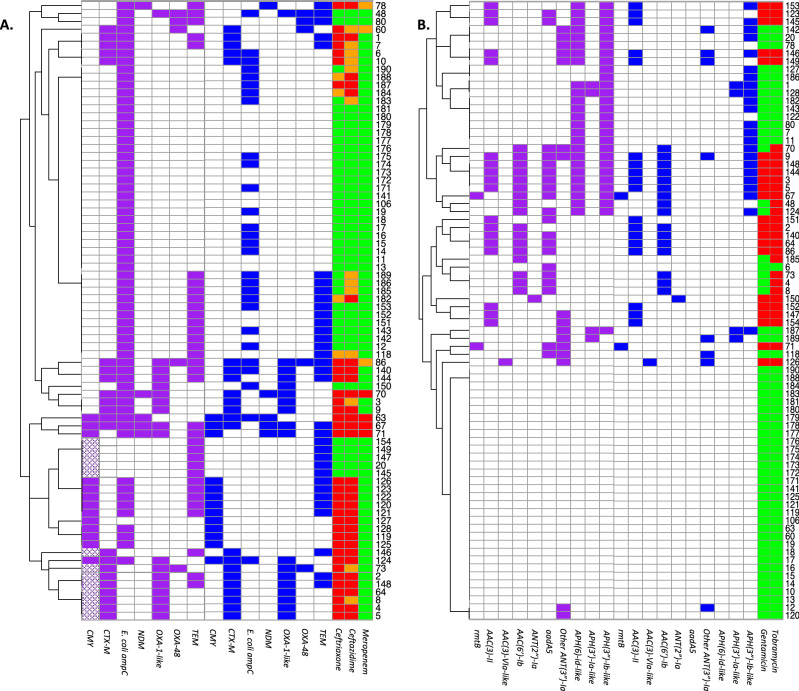
The carbapenemases NDM, OXA-48, KPC and VIM were detected at the protein level in all isolates carrying the corresponding genes. Similar to these carbapenemases, the extended spectrum beta-lactamase (ESBL) CTX-M and the beta-lactamases TEM and OXA-1 (Tables 1, 2 and Fig. 3) were also detected in all isolates carrying corresponding genes. Some of the other beta-lactamases were not always detected with LC–MS/MS. This was the case for SHV/LEN which was only detected in 30 of the 91 K. pneumoniae isolates with an encoding gene. Furthermore, presence of blaCMY-132-like genes in 13 E. coli isolates, and presence of blaDHA and blaOXA-9-like genes in 3 and 20 K. pneumoniae isolates was predicted by the resistance gene identifier, however, the corresponding proteins were not detected with MS. Additional analysis of WGS data showed that these genes were either partially present or were less than 80% similar to their reference genes. The proteins of the beta-lactamases OKP-B and LAP-2 were not detected in any of the isolates containing these genes. A peculiar result was obtained for the chromosomally encoded blaAmpC in E. coli. It is generally accepted that all E. coli isolates carry a chromosomally encoded blaAmpC gene which is in most cases not expressed or minimally expressed19,20. However, in our selected isolates, blaAmpC genes were only detected in 64 of the 78 E. coli isolates. In 28 of these 64 isolates, AmpC proteins were detected as well.
Table 1.
Presence of genes and proteins of beta-lactamases, 16S ribosomal RNA methyltransferases, aminoglycoside modifying enzymes and quinolone resistance proteins in the 78 analysed E. coli.
| Number of isolates with AMR gene | Number of isolates with AMR gene and protein | Proportion of isolates in which AMR gene was detected at the protein level (%) | |
|---|---|---|---|
| CMYa | 26 | 13 | 50 |
| CTX-M | 22 | 22 | 100 |
| Escherichia coli ampC | 64 | 28 | 44 |
| NDM | 5 | 5 | 100 |
| OXA-1-like | 18 | 18 | 100 |
| OXA-48 | 5 | 5 | 100 |
| TEM | 34 | 34 | 100 |
| RmtB | 2 | 2 | 100 |
| AAC(3)-II | 18 | 18 | 100 |
| AAC(3)-VIa-like | 1 | 1 | 100 |
| AAC(6′)-Ib | 17 | 16 | 94 |
| ANT(2″)-Ia | 1 | 1 | 100 |
| aadA5b | 20 | 0 | 0 |
| Other ANT(3″)-Ia | 16 | 8 | 50 |
| APH(6)-Id-like | 25 | 0 | 0 |
| APH(3′)-Ia-like | 4 | 4 | 100 |
| APH(3″)-Ib-like | 28 | 23 | 82 |
| QnrS1 | 2 | 0 | 0 |
Origin and nomenclature of beta-lactamase resistance genes and aminoglycoside modifying enzymes are described in the publication of Jacoby, and the publication of Ramirez and Tolmasky, respectively21,22.
aPresence of a CMY-132-like gene was predicted in 13 isolates using the Resistance Gene Identifier. However, these genes had less than 80% similarity to CMY-132. Furthermore, a CMY protein was not detected by MS in any of these 13 isolates.
bAlthough aadA5 also belongs to the ANT(3″)-Ia group, the protein sequence is quite distinct from the other ANT(3″)-Ia enzymes detected in this study. Therefore, the distinction between aadA5 and other ANT(3″)-Ia enzymes was made.
Table 2.
Presence of genes and proteins of beta-lactamases, 16S ribosomal RNA methyltransferases, aminoglycoside modifying enzymes, quinolone resistance proteins and OqxAB efflux pumps in the 109 analysed K. pneumoniae.
| Number of isolates with AMR mechanism detected in genome | Number of isolates with AMR mechanism in both genome and proteome | Proportion of isolates in which AMR gene was detected at the protein level (%) | |
|---|---|---|---|
| CMY | 8 | 8 | 100 |
| CTX-M | 50 | 50 | 100 |
| DHAa | 5 | 2 | 40 |
| KPC | 22 | 22 | 100 |
| LAP-2 | 1 | 0 | 0 |
| NDM | 18 | 18 | 100 |
| OKP-A | 2 | 1 | 50 |
| OKP-B | 5 | 0 | 0 |
| OXA-1 | 31 | 31 | 100 |
| OXA-9-likeb | 29 | 5 | 17 |
| OXA-48 | 20 | 20 | 100 |
| SHV/LEN | 91 | 30 | 33 |
| TEM | 42 | 42 | 100 |
| VIM | 13 | 13 | 100 |
| ArmA | 8 | 8 | 100 |
| RmtB | 1 | 1 | 100 |
| RmtC-like | 5 | 4 | 80 |
| RmtF | 2 | 2 | 100 |
| AAC(3)-II | 35 | 35 | 100 |
| AAC(3)-IV | 2 | 2 | 100 |
| AAC(3)-VIa-like | 2 | 2 | 100 |
| AAC(6′)-Ib | 37 | 37 | 100 |
| AAC(6′)-IIc | 1 | 0 | 0 |
| ANT(2″)-Ia | 4 | 0 | 0 |
| ANT(3″)-Ia | 60 | 30 | 50 |
| APH(4)-Ia | 2 | 2 | 100 |
| APH(6)-Id-like | 39 | 5 | 13 |
| APH(3′)-Ia-like | 36 | 36 | 100 |
| APH(3′)-VI | 1 | 0 | 0 |
| APH(3″)-Ib-like | 39 | 36 | 92 |
| QnrA1 | 5 | 5 | 100 |
| QnrB | 16 | 11 | 69 |
| QnrS | 8 | 0 | 0 |
| OqxA | 108 | 32 | 30 |
| OqxB | 107 | 32 | 30 |
Origin and nomenclature of beta-lactamase resistance genes and aminoglycoside modifying enzymes are described in the publication of Jacoby, and the publication of Ramirez and Tolmasky, respectively21,22.
aDHA genes were detected in five isolates. However, in three of these isolates only 70% of the sequence was covered. The corresponding proteins were not detected by MS.
bOXA-9-like genes were detected in 29 isolates. However in 20 of these isolates only 40% of the sequence was covered. The corresponding protein were not detected by MS in any of these 20 isolates but also not in four of the nine isolates with a completely covered gene.
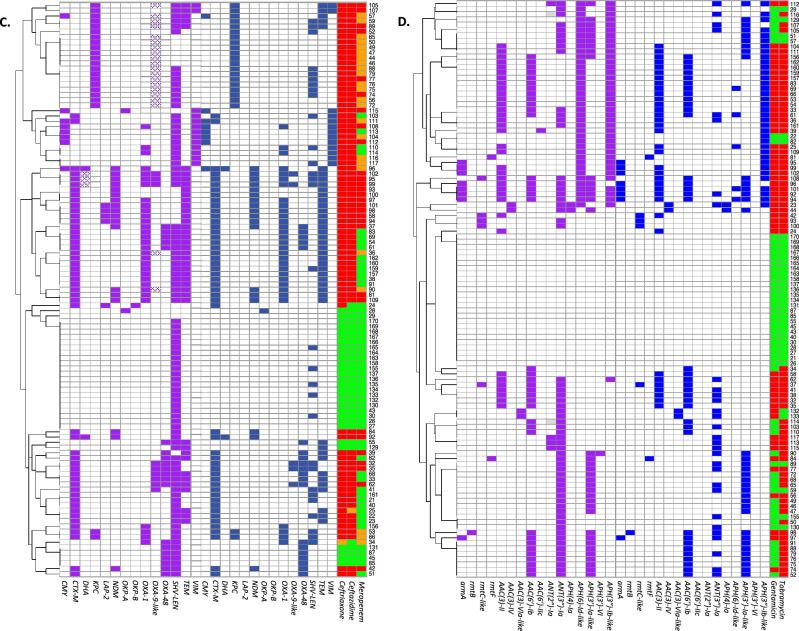
Figure 3.
An overview of the different resistance mechanisms at the gene and protein level in the studied E. coli and K. pneumoniae isolates. The presence of beta-lactamase genes and proteins in the 78 E. coli and 109 K. pneumoniae isolates are shown in (A, C). The presence of 16S ribosomal RNA methyltransferase (16S-RMTase) and aminoglycoside modifying enzyme (AME) genes and proteins are shown in (B, D) for E. coli and K. pneumoniae, respectively. For each isolate, present genes were depicted in purple, proteins in blue and susceptible, intermediate and resistant phenotypes in green, orange, and red, respectively. Genes that were partly present and genes that were less than 80% similar to their reference genes were depicted with a dotted pattern. Isolates were clustered based on presence of the depicted AMR genes.
To correlate the presence of the various beta-lactamases with their phenotypes, we classified isolates arbitrarily based on their MICs for 3rd generation cephalosporins and meropenem into “wild type” (WT) combined with small-spectrum beta-lactamase and/or oxacillinase-producing isolates (PEN/OXA), ESBL or AmpC-producing isolates, chromosomal E. coli AmpC-producing isolates (for E. coli only), or carbapenemase producing isolates (CPE). For E. coli, the isolates susceptible to 3rd generation cephalosporins and with meropenem MICs below the epidemiological cut-off value (ECOFF; www.eucast.org) of 0.125 mg/L did either not have any acquired beta-lactamases (n = 21) or had a small spectrum beta-lactamase such as TEM (n = 11) or OXA-1 (n = 1). Remarkably, in 12 of these 33 3rd generation susceptible isolates, the chromosomally encoded AmpC enzyme was still detected at the protein level without compromising the susceptibility to ceftazidime or ceftriaxone. In the isolates that were not susceptible to ceftazidime or ceftriaxone but with meropenem MICs ≤ 0.125 mg/L (n = 35), the ESBL CTX-M (n = 16), a CMY enzyme (n = 10) or only a chromosomal AmpC (n = 9) were detected. In the nine E. coli isolates in which only chromosomal AmpC proteins were detected, this resulted in ceftazidime MICs of 4–8 mg/L and ceftriaxone MICs < 2 mg/L except for one isolate with a ceftriaxone MIC of 4 mg/L. In the ten meropenem-resistant E. coli isolates (CPE) either OXA-48 or NDM proteins were detected, sometimes in combination with CMY and/or CTX-M (Table 3). Remarkably, in two OXA-48-positive isolates with an MIC of 8 mg/L for meropenem, the porin OmpC could not be demonstrated while in the remaining three isolates with MICs of 0.5–2 mg/L, OmpC was detected. This suggests a difference in OmpC abundance which may explain this difference in meropenem MICs.
Table 3.
Seventy-eight E. coli isolates classified according to their beta-lactamase phenotype including the detected resistance mechanisms against beta-lactams and aminoglycosides by LC–MS/MS.
| Phenotype | n | Antibiotics | PEN/OXA | ESBL | AmpC | CPE | 16S-RMTase | AME | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRX | CAZ | MEM | GEN | TOB | CIP | TEM | OXA-1 | CTX-M | CMY | cAmpC | OXA-48 | NDM | RmtB | AAC(3)-II | AAC(3)-VI | AAC(6′)-Ib | ANT(2″)-Ia | ||
| WT/PEN | 25 | S | S | S | S | S | S/R | 4 | – | – | – | 10 | – | – | – | – | – | – | – |
| WT/PEN/AME | 8 | S | S | S | R | R | S/R | 7 | 1 | – | – | 2 | – | – | – | 7 | – | – | 1 |
| ESBL/AmpC | 12 | S/R | S/R | S | S | S | S/R | 6 | – | 4 | 7 | 2 | – | – | – | – | – | – | – |
| ESBL/AmpC/AME | 14 | R | R | S | S/R | R | S/R | 7 | 11 | 12 | 3 | 2 | – | – | – | 10 | 1 | 11 | – |
| AmpC | 6 | S/R | R | S | S | S | S | 1 | – | – | – | 6 | – | – | – | – | – | – | – |
| AmpC/Quin | 3 | S | R | S | S | S/R | R | 3 | – | – | – | 3 | – | – | – | – | – | – | – |
| CPE | 3 | S/R | S/R | S/R | S | S | S | 2 | – | 1 | – | – | 2 | 1 | – | – | – | – | – |
| CPE/Quin | 7 | S/R | S/R | S/R | S/R | S/R | R | 4 | 6 | 5 | 3 | 3 | 3 | 4 | 2 | 1 | – | 5 | – |
Isolates were classified based on their susceptibility (S) or resistance (R) to ceftriaxone (CRX), ceftazidime (CAZ), meropenem (MEM), gentamicin (GEN), tobramycin (TOB) and ciprofloxacin (CIP) into wild type (WT) isolates and small spectrum penicillinase (PEN) and/or oxacillinase-producing (OXA) isolates, extended-spectrum beta-lactamase producers (ESBL) in combination with isolates producing AmpC beta-lactamases (AmpC), isolates that only produced E. coli chromosomal AmpC, or carbapenemase producing Enterobacterales (CPE). Isolates were further divided based on aminoglycoside resistance, mostly caused by aminoglycoside modifying enzymes (AME), and/or quinolone resistance (Quin). Small spectrum beta-lactamases/oxacillinases included TEM and OXA-1. ESBL and AmpC were represented by CTX-M, CMY and E. coli chromosomal AmpC. Carbapenemase producing Enterobacterales (CPE) included OXA-48 and New Delhi Metallo beta-lactamase (NDM) producing isolates. Mechanisms of aminoglycoside resistance included the 16S rRNA methyltransferase (rmtB), the acetyltransferases AAC(3)-II, AAC(3)-VI and AAC(6′)-Ib, and the nucleotidyltransferase ANT(2″)-I.
For the K. pneumoniae isolates, a similar analysis was performed. We tried to explain the different phenotypes by analyzing the beta-lactamases detected at the protein level (Table 4). In all 29 isolates susceptible to 3rd generation cephalosporins, no enzymes were detected with substrate specificity to 3rd generation cephalosporins except for one isolate in which the ESBL SHV-27 was detected. This isolate still had MICs of 0.25 mg/L or lower for ceftazidime and ceftriaxone. Of the 3rd generation cephalosporin susceptible isolates, five isolates had meropenem MICs of 0.25–1 mg/L and were positive for OXA-48 at both the genome and the proteome level. In the group of 3rd generation cephalosporin resistant isolates with meropenem MIC’s ≤ 0.25 mg/L, CTX-M was detected (n = 12). In addition, in some of these isolates TEM-1 and OXA-1 were also present. The remaining group consisting of 68 isolates all displayed MICs above the screening breakpoint for meropenem and in the majority of these isolates, one carbapenemase was demonstrated per isolate. The isolates in which either KPC or VIM was detected showed a wide range of MICs suggesting that additional resistance mechanisms are acting in concert to affect the meropenem MICs. The isolates in which OXA-48 enzymes were detected also showed a wide range in meropenem MICs. Nevertheless, all OXA-48 enzymes were detected by LC–MS/MS. In one isolate with an MIC of 4 mg/L for meropenem, no carbapenemases were detected but instead CTX-M, TEM and OXA-1 were demonstrated while the two major porins OmpK35 and OmpK36 were not detected at the protein level. The combination of these mechanisms may explain the increased MIC to meropenem. In general, LC–MS/MS was able to demonstrate the presence of the proteins conferring resistance to meropenem and 3rd generation cephalosporins, even in organisms harboring mechanisms that were hard to detect by phenotypic assays, i.e. in isolates displaying low MICs for their indicator antibiotics.
Table 4.
One hundred and nine K. pneumoniae isolates classified according to their beta-lactamase phenotype including the detected resistance mechanisms against beta-lactams and aminoglycosides by LC–MS/MS.
| Phenotype | n | Antibiotics | PEN/OXA | ESBL | AmpC | CPE | 16S-RMTase | AAC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRX | CAZ | MEM | GEN | TOB | CIP | TEM | SHV | OXA-1 | OXA-9 | CTX-M | CMY | DHA | OXA-48 | NDM | KPC | VIM | AAC(3) | AAC(6′) | |||
| WT/PEN/OXA | 27 | S | S | S | S | S | S/R | 2 | 4 | – | – | – | – | – | 5 | – | – | – | – | – | – |
| WT/PEN/OXA/AME | 2 | S | S | S | R | S | S | – | 1 | – | – | – | – | – | – | – | – | – | – | 2 | – |
| ESBL/AmpC | 2 | R | R | S | S | S | S/R | 1 | 2 | – | – | 2 | – | – | – | – | – | – | – | – | – |
| ESBL/AmpC/AME | 10 | R | S/R | S | R | S/R | S/R | 7 | 2 | 6 | – | 10 | – | – | – | – | – | – | – | 9 | 6 |
| CRE/Quin | 4 | R | R | S/R | S | S | R | 3 | 2 | – | – | 1 | 1 | – | – | – | 3 | – | – | – | – |
| CRE/AME | 6 | R | R | S/R | S/R | S/R | S/R | 1 | 1 | 2 | – | 2 | 1 | – | 2 | – | – | 4 | – | – | 2 |
| CRE/AME/Quin | 58 | R | R | S/R | S/R | R | R | 28 | 18 | 23 | 5 | 35 | 6 | 2 | 13 | 18 | 19 | 9 | 15 | 28 | 29 |
Isolates were classified based on their susceptibility (S) or resistance (R) to ceftriaxone (CRX), ceftazidime (CAZ), meropenem (MEM), gentamicin (GEN), tobramycin (TOB) and ciprofloxacin (CIP) into wild type (WT) isolates and small spectrum penicillinase (PEN) and/or oxacillinase-producing (OXA) isolates, extended-spectrum beta-lactamase producers (ESBL) in combination with isolates producing AmpC beta-lactamases (AmpC), or carbapenem resistant Enterobacterales (CRE). All CRE isolates had MEM MIC’s ≥ 0.5 mg/L. Isolates were further divided based on aminoglycoside resistance, mostly caused by aminoglycoside modifying enzymes (AME) and/or quinolone resistance (Quin). Small spectrum beta-lactamases/oxacillinases included TEM, SHV, OXA-1 and OXA-9. ESBL and AmpC were represented by CTX-M, CMY and DHA. Carbapenemase producing Enterobacterales (CPE) included OXA-48, NDM, KPC and VIM positive isolates. Mechanisms of aminoglycoside resistance included the 16S rRNA methyltransferases ArmA, RmtB, RmtC and RmtF and the acetyltransferase group AAC(3) which included AAC(3)-II, AAC(3)-IV and AAC(3)-VI and the acetyltransferase AAC(6′)-Ib.
Aminoglycoside resistance
Aminoglycoside resistance in Enterobacterales is mainly caused by aminoglycoside modifying enzymes (AMEs) and/or the presence of 16S ribosomal RNA methyltransferases (16S-RMTases)21,23. In the examined isolates, genes encoding four different 16S-RMTases and 12 different AME groups were detected. All 16S-RMTase genes, i.e. armA, rmtB, rmtC and rmtF were detected at the protein level by MS except for rmtC in one isolate. Of the AMEs, most acetyltransferases (AAC) were detected by MS while this varied for adenyltransferases (ANT) and phosphotransferases (APH). Results for all 16S-RMTases and AMEs are shown in Tables 1, 2 and Fig. 3.
Previous studies have shown major differences in substrate specificity between the different aminoglycoside modifying enzymes21. This was also confirmed in the present study as for instance the presence of only ANT(3″)-Ia, APH(6)-Id-like, or APH (3″)-Ib-like enzymes in E. coli did not result in resistance to gentamicin or tobramycin. In contrast, the 18 isolates with AAC(3)-II, the isolate with AAC(3)-VIa-like and the isolate with ANT(2″)-Ia, all showed MICs > 8 mg/L for gentamicin and MICs ≥ 4 mg/L for tobramycin, indicating resistance to both antibiotics. Furthermore, the 16 isolates with only AAC(6′)-Ib all had MICs ≥ 8 mg/L for tobramycin and MICs of 1 or 2 mg/L for gentamicin. In one isolate an AAC(6′)-Ib9-like gene resulted in an MIC of 8 mg/L for tobramycin but the corresponding protein was not detected. The two isolates in which the 16S-RMTase RmtB was detected had MICs > 8 mg/L for both gentamicin and tobramycin. All 49 remaining isolates with none of these resistance mechanisms were susceptible to gentamicin and tobramycin.
For K. pneumoniae, generally similar observations were made as for E. coli (Table 4). All K. pneumoniae isolates in which a 16S-RMTase (n = 16) was detected had MICs > 8 mg/L for both gentamicin and tobramycin. All isolates in which an AAC(3) enzyme (n = 39) was detected had MICs > 8 mg/L for gentamicin and usually also MICs > 8 mg/L for tobramycin (n = 34). However, in many of these isolates AAC(6′)-Ib was detected as well. In absence of other AACs or 16S-RMTases, the presence of only AAC(6′)-Ib (n = 11) resulted in MICs > 8 mg/L for tobramycin and MICs ≤ 2 mg/L for gentamicin, except for one isolate with a gentamicin MIC of 4 mg/L. In 4 isolates an ant(2″)-Ia gene was identified which conferred resistance to both gentamicin and tobramycin. However, the corresponding protein was not detected. In 12 of the 72 K. pneumoniae isolates resistant to tobramycin, no AME or 16S-RMTase genes were identified that could explain the corresponding phenotype. However in 9 of these 12 isolates, AAC(6′)-Ib(-like) proteins were detected. This indicated the presence of this enzyme or a similar protein which most likely explains the tobramycin resistance.
Fluoroquinolone resistance
Fluoroquinolone resistance in E. coli and K. pneumoniae can be caused by (a combination of) target site mutations, enzymes modifying fluoroquinolones, physical blocking of the target site by Qnr proteins, and presence or increased expression of specific efflux pumps10,24.
In the analyzed isolates, QnrA was detected by MS in five out of five isolates with encoding genes and QnrB in 11 out of 16 isolates. Remarkably, QnrS was not detected in any of the ten isolates with encoding genes (Tables 1 and 2). The AME AAC(6′)-Ib-cr which also acetylates ciprofloxacin in addition to aminoglycosides was detected in all isolates (41/41). The genes oqxA and oqxB were detected in 108 and 107 K. pneumoniae isolates, respectively, but in none of the E. coli isolates. The OqxAB protein complex was detected in 29 K. pneumoniae isolates while only OqxA was detected in three isolates and only OqxB in three other isolates.
We assessed whether the detected fluoroquinolone resistance mechanisms could explain increased MICs to ciprofloxacin. In the 36 E. coli isolates with an MIC ≤ the ECOFF of 0.064 mg/L no resistance mechanisms were detected. In contrast, all 33 resistant E. coli isolates with an MIC ≥ 1 mg/L had at least one mutation linked to fluoroquinolone resistance in gyrA. Furthermore, 29 of these isolates had at least one mutation in parC. In addition, in 16 isolates AAC(6′)-Ib-cr was detected, and in 2 isolates a qnrS gene was identified. Finally, 9 isolates had an MIC of 0.125 or 0.25 mg/L which was above the ECOFF of 0.064 mg/L but still within the susceptible range. In eight of these isolates at least one mutation in gyrA was identified, corresponding with the moderately increased MICs.
Of the 109 K. pneumoniae isolates, 73 isolates were resistant with an MIC ≥ 1 mg/L. In 62 of these isolates, one or more mutations were identified in parC and in 37 isolates one or more mutations in gyrA. Furthermore, in 28 isolates the OqxAB complex was detected and in 23 isolates AAC(6′)-Ib-cr. In 15 isolates, a qnrB gene was identified, in 7 a qnrS gene, and in 5 a qnrA gene. Thirty isolates had MICs ≤ ECOFF of 0.125 mg/L and in two of these isolates AAC(6′)-Ib-cr was detected. In the 28 remaining isolates, none of these resistance mechanisms were identified, as was also the case for two isolates with an MIC of 0.25 mg/L. Finally, a qnrB gene, a qnrS gene, a mutation in gyrA and the OqxAB protein were each identified once in the four intermediate (MIC of 0.5 mg/L) isolates, thereby explaining these moderately increased MICs.
Porin analysis
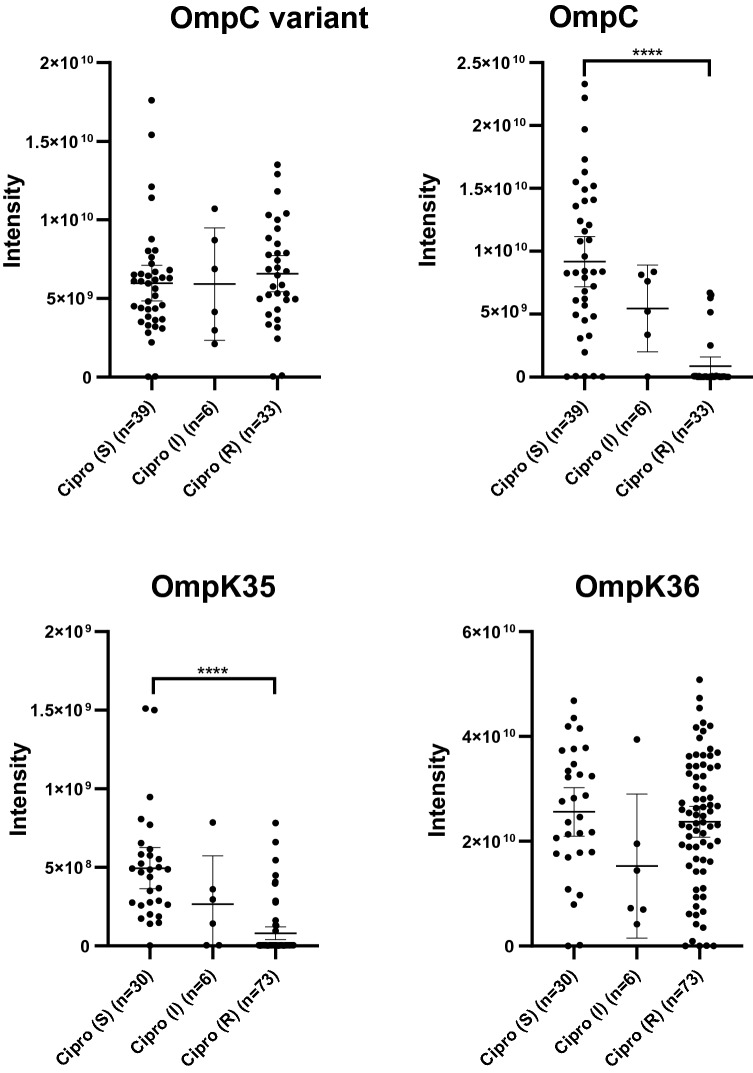
The presence of single resistance mechanisms can already cause resistance to certain antibiotic classes. However, secondary mechanisms often contribute to increase MICs past the breakpoint used to determine (non-)susceptibility. For instance, a decrease in porin expression acts in concert with enzymes such as beta-lactamases. In the present study, OmpF abundance in E. coli was negatively correlated with increasing MICs to each of the four antibiotic classes studied, but was only found to have a significantly lower abundance in aminoglycoside resistant isolates than in aminoglycoside susceptible isolates. Of the other major porin OmpC, a variant was identified that was significantly less abundant in cephalosporin, aminoglycoside and ciprofloxacin resistant isolates. In contrast, the “regular” OmpC porin was not correlated to resistance (Fig. 4 and Supplementary Fig. 1). Interestingly, the maltoporin LamB was significantly less abundant in meropenem resistant isolates. In K. pneumoniae, OmpK35 (orthologue of OmpF) was significantly less abundant in isolates resistant to meropenem, 3rd generation cephalosporins, aminoglycosides or ciprofloxacin. As the majority of these isolates were resistant to more than one class of antibiotics, OmpK35 abundance could not be correlated to resistance against one specific antibiotic class. OmpK36 (orthologue of OmpC) abundance was not significantly correlated to resistance to any of the antibiotics (Fig. 4). The third major porin, i.e. PhoE was not detected in the isolates of either E. coli or K. pneumoniae which is to be expected as isolates were cultured under general culture conditions without limiting phosphate concentrations.
Figure 4.
Porin abundance was compared between ciprofloxacin susceptible, intermediate and resistant E. coli and K. pneumoniae isolates. Signal intensities for the different porins are shown in arbitrary units for the “regular” OmpC and the OmpC variant in E. coli, and for OmpK35 and OmpK36 in K. pneumoniae. ****p-value < 0.0001 (Ordinary one-way Anova, Turkey’s multiple comparisons test).
Discovery-based analysis
In addition to analysis of specific AMR mechanisms, a discovery-based analysis was performed to identify protein groups which were significantly correlated with resistance to meropenem, third generation cephalosporins, aminoglycosides, ciprofloxacin or a combination of these antibiotics/classes. To compare protein abundance between resistant and susceptible isolates, MS data was log2 transformed and imputation was applied for missing values. As most isolates that were resistant to one antibiotic class were also resistant to other antibiotic classes, most proteins could not be correlated to resistance against one specific class. In the 78 E. coli isolates, a total of 7951 protein groups were detected of which 80 groups were significantly more abundant in the resistant isolates. In addition, 46 protein groups were significantly less abundant in the resistant isolates. In the 109 K. pneumoniae isolates, 8648 protein groups were detected of which 208 groups were significantly more abundant in the resistant isolates. Furthermore, 82 protein groups were significantly less abundant in the resistant isolates. Data on all detected protein groups including their measured intensities and their correlation to resistance to each of the antibiotic classes is available in the “Supplementary datasets”.
The 46 protein groups which were more than an arbitrary four times as abundant in isolates resistant to any of the antibiotic classes are shown in Tables 5 and 6. Of these, 23 protein groups were already curated as AMR mechanisms and consisted mostly of beta-lactamases or other antibiotic altering or degrading enzymes. The protein groups NDM and CTX-M in E. coli, and KPC, CTX-M and AAC(6′)-Ib in K. pneumoniae were more than 20 times as abundant in resistant isolates. Some resistance mechanisms correlated more strongly to another antibiotic class than to the class that they primarily affect due to co-presence of other resistance mechanisms. For instance, in E. coli, the 16S-RMTase RmtB was found to correlate the best with meropenem resistance as the two isolates that produced RmtB also produced NDM. Similarly, aminoglycoside resistant E. coli isolates often produced TEM and OXA-1, while ciprofloxacin resistant K. pneumoniae isolates often produced TEM as well.
Table 5.
All protein groups which were more than four times as abundant in resistant E. coli isolates compared to susceptible E. coli isolates.
| Protein | Most correlated to resistance to | P-value | Fold change | Curated in CARD |
|---|---|---|---|---|
| Class C extended-spectrum beta-lactamase CMY-42 | 3rd gen cephalosporins | < 0.001 | 6.13 | Yes |
| Class A extended-spectrum beta-lactamase CTX-M-15 | 3rd gen cephalosporins | < 0.001 | 24.77 | Yes |
| Carbapenem-hydrolyzing class D beta-lactamase OXA-48 | Meropenem | < 0.001 | 8.48 | Yes |
| 16S rRNA (guanine(1405)-N(7))-methyltransferase RmtB1 | Meropenem | < 0.001 | 5.47 | Yes |
| Subclass B1 metallo-beta-lactamase NDM-5 | Meropenem | < 0.001 | 61.22 | Yes |
| YkgJ family cysteine cluster protein | Meropenem | < 0.001 | 4.71 | No |
| Polysaccharide export protein | Meropenem | < 0.001 | 6.09 | No |
| Pseudouridine-5′-phosphate glycosidase | Meropenem | < 0.001 | 5.20 | No |
| H-NS histone family protein | Meropenem | < 0.001 | 14.60 | Yes |
| Aminoglycoside N-acetyltransferase AAC(3)-IIa | Aminoglycosides | < 0.001 | 5.82 | Yes |
| Class A beta-lactamase TEM-210 | Aminoglycosides | < 0.001 | 7.62 | Yes |
| Mph(A) family macrolide 2′-phosphotransferase | Aminoglycosides | < 0.001 | 13.38 | Yes |
| OXA-1 family class D beta-lactamase | Aminoglycosides | < 0.001 | 13.52 | Yes |
| sce7725 family protein | Aminoglycosides | < 0.001 | 4.14 | No |
| Superoxide dismutase [Fe] | Aminoglycosides | < 0.001 | 7.01 | No |
| Ketobutyrate formate-lyase/pyruvate formate-lyase | Aminoglycosides | < 0.001 | 5.67 | No |
| Phosphoenolpyruvate-protein phosphotransferase PtsI | Aminoglycosides | < 0.001 | 4.89 | Yes |
| 50S ribosomal protein L1 | Aminoglycosides | 0.0019 | 4.51 | No |
| Fluoroquinolone-acetylating aminoglycoside 6′-N-acetyltransferase AAC(6′)-Ib-cr5 | Ciprofloxacin | < 0.001 | 12.10 | Yes |
| NADP-dependent phosphogluconate dehydrogenase | Ciprofloxacin | < 0.001 | 6.89 | No |
| NADP-dependent phosphogluconate dehydrogenase | Ciprofloxacin | < 0.001 | 5.12 | No |
| UDP-glucose 6-dehydrogenase | Ciprofloxacin | < 0.001 | 4.60 | No |
| Plasmid-partitioning protein SopA | Ciprofloxacin | < 0.001 | 5.11 | No |
Table 6.
All protein groups which were more than 4 times as abundant in resistant K. pneumoniae isolates compared to susceptible K. pneumoniae isolates.
| Protein | Most correlated to resistance to | P-value | Fold change | Curated in CARD |
|---|---|---|---|---|
| Aminoglycoside O-phosphotransferase APH(3″)-Ib | 3rd gen cephalosporins | < 0.001 | 5.70 | Yes |
| Class A extended-spectrum beta-lactamase CTX-M-15 | 3rd gen cephalosporins | < 0.001 | 41.51 | Yes |
| Heat shock survival AAA family ATPase ClpK | 3rd gen cephalosporins | 0.002 | 4.00 | No |
| Subclass B1 metallo-beta-lactamase NDM-1 | Meropenem | < 0.001 | 7.29 | Yes |
| Class A extended-spectrum beta-lactamase SHV-5 | Meropenem | < 0.001 | 8.74 | Yes |
| Type A-1 chloramphenicol O-acetyltransferase | Meropenem | < 0.001 | 9.69 | Yes |
| Aminoglycoside O-phosphotransferase APH(3′)-Ia | Meropenem | < 0.001 | 10.97 | Yes |
| Carbapenem-hydrolyzing class A beta-lactamase KPC-2 | Meropenem | < 0.001 | 20.03 | Yes |
| Ribonuclease E | Meropenem | < 0.001 | 4.75 | No |
| Type I restriction endonuclease subunit R | Meropenem | < 0.001 | 4.70 | No |
| Porin (not otherwise specified) | Meropenem | < 0.001 | 4.07 | No |
| Trigger factor | Meropenem | < 0.001 | 6.31 | No |
| Hypothetical protein | Meropenem | < 0.001 | 4.72 | No |
| Outer membrane protein assembly factor BamE | Meropenem | < 0.001 | 4.19 | No |
| C-Lysozyme inhibitor | Meropenem | < 0.001 | 4.86 | No |
| Aldo/keto reductase family oxidoreductase | Meropenem | < 0.001 | 4.26 | No |
| SAM-dependent DNA methyltransferase | Meropenem | < 0.001 | 4.73 | No |
| DUF305 domain-containing protein | Meropenem | < 0.001 | 12.18 | No |
| Aminoglycoside N-acetyltransferase AAC(3)-IIa | Aminoglycosides | < 0.001 | 6.05 | Yes |
| Fluoroquinolone-acetylating aminoglycoside 6′-N-acetyltransferase AAC(6′)-Ib-cr5 | Aminoglycosides | < 0.001 | 32.97 | Yes |
| Multidrug efflux RND transporter periplasmic adaptor subunit OqxA | Ciprofloxacin | < 0.001 | 5.04 | Yes |
| Class A broad-spectrum beta-lactamase TEM-1 | Ciprofloxacin | < 0.001 | 9.10 | Yes |
| LPS assembly lipoprotein LptE | Ciprofloxacin | < 0.001 | 4.12 | No |
In addition to the protein groups curated in CARD, 23 other protein groups were more than four times as abundant in isolates resistant to any of the antibiotic classes. The majority of these protein groups were variants of protein groups not correlated with resistance. Four protein groups were an exception and had little to no variant groups. These were the YkgJ family cysteine cluster protein, the sce7725 family protein and the plasmid-partitioning protein SopA in E. coli, and the outer membrane protein assembly factor BamE in K. pneumoniae.
Discussion
A thorough understanding of antimicrobial resistance is key for both the development of antibiotics and diagnostic tools. Antimicrobial resistance is mostly studied using growth-inhibition methods and DNA detection and sequencing techniques. However, as new AMR protein detection methods are developed that are based on MS9,15,16, it is important to know which resistance mechanisms can be detected at the protein level and if they can predict phenotypic resistance as well. In the current study, we performed a systematic analysis of meropenem, third generation cephalosporin, aminoglycoside, and ciprofloxacin resistance in 187 selected E. coli and K. pneumoniae isolates harboring different antibiotic resistance mechanisms. The majority of the studied isolates was unique as was determined by means of cgMLST, which suggests that our findings are generalizable for both bacterial species. We demonstrated that the proteins of different antimicrobial resistance mechanisms can be detected using a proteogenomic approach with bottom-up LC–MS/MS. Especially beta-lactamases, 16S-RMTases, and AACs were detected in the proteome with high sensitivity. Remarkably, some other mechanisms were not detected at all with LC–MS/MS, or they were only detected in a minority of the isolates with an encoding gene. For some AMR mechanisms, such as OXA-9-like, CMY-132-like or DHA, this could be explained by the presence of partial genes which did not lead to functional proteins and did also not confer resistance. Such genes might not be transcribed, or the resulting proteins might be degraded at an early stage. Other proteins which were not detected or only in a few isolates were aadA5, APH(6)-Id-like and QnrS. The low detection rate of these mechanisms might imply that the proteins are present in quantities below the detection limit, or that they are altered by post translational modifications. Alternatively, the encoding genes might only be transcribed and translated under certain conditions.
Although not all AMR mechanisms were detected at the protein level, resistance could be explained by the detected proteins for most of the selected isolates. In all isolates resistant to meropenem, a carbapenemase was detected. Furthermore, in all isolates resistant to 3rd generation cephalosporins, ESBLs, AmpC enzymes and/or carbapenemases were detected. Gentamicin and tobramycin resistance could be explained by the proteogenomic approach in 100% and 97% of the E. coli isolates, respectively. These numbers were lower for K. pneumoniae, the resistant phenotype could be explained in 89% of the gentamicin resistant isolates and 76% of the tobramycin resistant isolates. The AMR gene that resulted in tobramycin resistance could not be identified in 12 K. pneumoniae isolates even though AAC(6′)-Ib(-like) proteins were detected in nine of these isolates after additional analysis. A possible explanation for this discrepancy could be the use of only short-read sequencing resulting in incomplete coverage of genomes. This could also explain why blaAmpC and blaSHV genes were not detected in all of the E. coli and K. pneumoniae isolates20,25. Still, more than 95% of the core genes were detected in each isolate. Ciprofloxacin resistance in the studied isolates could be explained by mutations in gyrA and parC, and by the presence of AAC(6′)-Ib-cr enzymes, OqxAB efflux pumps and qnr genes. In the current study, we did not correlate mutations at the DNA level to the detected peptides as a targeted MS approach is more suitable to detect these key amino acid substitutions as demonstrated by Hassing et al.26.
In addition to the analysis of resistance mechanisms curated in CARD, we analyzed porin abundance as a decrease in porins or complete loss of a porin contributes to resistance27. For instance, a total lack of OmpC is associated with resistance to quinolones28, and beta-lactams27,29. This was not demonstrated in the current study but instead a variant of OmpC was detected that was significantly less abundant in E. coli isolates resistant to 3rd generation cephalosporins, aminoglycosides or ciprofloxacin. This OmpC variant differed substantially compared to the “regular” OmpC and differences in multiple regions may affect membrane permeability. For instance, the insertion of Gln in loop L3 (position 142 of the alignment) could affect the pore diameter27. Additional experiments are required to assess which of the structural differences affect permeability to antibiotics the most. Furthermore, we demonstrated that both OmpF in E. coli and its orthologue OmpK35 in K. pneumoniae were less abundant in resistant isolates. This finding corresponds with previous literature in which the absence of OmpF, or expression of the less permeable OmpC instead of OmpF resulted in a reduced influx of beta-lactams and quinolones and a resulting increase in MICs10,27,30,31.
Furthermore, a discovery-based analysis was performed by which we identified several correlations of proteins to resistance. We found that the plasmid-partitioning protein SopA was significantly more abundant in ciprofloxacin resistant E. coli isolates. This protein plays a role in plasmid partitioning of F plasmids which are major carriers of acquired resistance genes in E. coli32,33. In K. pneumoniae, presence of the outer membrane protein assembly factor BamE was significantly correlated to resistance. Previously, Sikora et al. described BamE was not a vital protein for N. gonorrhoeae, but absence of BamE resulted in an altered cell envelope composition and an increase in antibiotic susceptibility34. We did not find links in the literature between antimicrobial resistance and the identified YkgJ family cysteine cluster protein or the sce7725 family protein. Nonetheless, none of the genes encoding these four proteins were located next to genes of curated AMR mechanisms indicating a (independent) correlation to resistance in the studied isolates. In addition to these protein groups, many other protein groups were identified that correlated to resistance (“Supplementary datasets”). Further experiments and analyses are required to assess if and how these protein groups are involved in antibiotic resistance.
Previous studies which applied discovery based-proteomics in E. coli and K. pneumoniae analyzed a single or a few isolates35–38, or focused on a specific mechanism39. In the current study, resistance against commonly used antibiotics within the beta-lactam, aminoglycoside, and fluoroquinolone groups was analyzed in a significant number of clinical E. coli and K. pneumoniae isolates by a combination of both LC–MS/MS and WGS. Altogether, our findings indicate that the majority of the known AMR proteins causing resistance to beta-lactams and aminoglycosides can be detected by bottom-up proteomics without prior exposure to antibiotics. The high detection rate of resistance mechanisms by MS was facilitated by the use of a protein database which was assembled using WGS data from all of the studied isolates. Unfortunately, publicly available protein databases such as UniProt are incomplete for resistance mechanisms and require the inclusion of proteins from many bacterial species which results in more false-positives and/or a decrease in sensitivity. The quality of the protein database affected the sensitivity and specificity of the current LC–MS/MS analyses and shows its evident potential provided that a concise and optimal database is used. The current findings supports research endeavors aiming to develop rapid protein detection methods for antimicrobial resistance testing, perhaps by using a shorter sample pre-treatment protocol and a more high-throughput LC–MS method. Furthermore, the extensive amount of multi-omics data generated in this study showed correlations between resistance and various proteins that were not yet described. Finally, this study shows LC–MS/MS is a different and complementary method which can be used to study antimicrobial resistance in detail.
Materials and methods
Bacterial isolates
Ethical approval was not required, as only stored bacterial isolates were used. A variety of different E. coli and K. pneumoniae isolates were obtained with most of the isolates being resistant to one or more of the antibiotics of interest. Altogether, 187 isolates were used of which 117 E. coli and K. pneumoniae isolates were obtained from the Erasmus MC collection. Of these, nine E. coli isolates were selected based on a phenotype which suggested hyperproduction of chromosomal AmpC (MICs for cefoxitin > 16 mg/L, MICs for ceftazidime of 4–8 mg/L and twofold higher than MICs for ceftriaxone)40. Furthermore, 10 E. coli isolates were selected that were known to carry a CMY gene and 23 E. coli and K. pneumoniae isolates were selected based on being resistant to gentamicin, ciprofloxacin or both. The remaining 75 isolates were either carbapenem resistant and/or 3rd generation cephalosporin resistant, or they were susceptible to both carbapenems and 3rd generation cephalosporins. In addition, 46 E. coli and K. pneumoniae isolates that were ESBL and/or carbapenemase-positive were obtained from the Dutch National Institute for Public Health and the Environment (RIVM), 13 predominantly VIM positive K. pneumoniae isolates were obtained from the National School of Public Health, Athens, Greece, and 11 predominantly OXA-48 positive K. pneumoniae isolates were obtained from the Regional Institute of Gastroenterology and Hepatology in Cluj Napoca, Romania. The MICs of all isolates are displayed in Supplementary Tables 1 and 2.
Culture protocol
Sub-cultured isolates stored at − 80 °C were thawed, cultured on Trypticase™ Soy Agar II plates with 5% sheep blood (Becton Dickinson, New Jersey, USA), and incubated overnight at 37 °C. Subsequently, one inoculation loop of bacteria was inoculated in 30 mL MH II broth and incubated overnight at 37 °C at 150 rpm. Next, the broth culture was centrifuged for 30 min at 4500g, and the pellet was washed with 10 mL phosphate-buffered saline. Subsequently, 6 mL phosphate-buffered saline was added, the samples were vortexed and 1 mL was transferred in each of six aliquots which were centrifuged for 5 min at 21,000g. The resulting six identical pellets per isolate were stored at − 80 °C. These pellets were used for AST, WGS and LC–MS/MS.
Identification and AST
All isolates were previously identified in our laboratory using the MALDI biotyper (Bruker, Billerica, USA). AST was performed using custom microdilution Sensititre® MIC susceptibility plates in accordance with the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, United States). Clinical breakpoints and ECOFFs of the EUCAST were applied for the general classification of the isolates. For the discovery-based analysis which included the porin analysis, different breakpoints were used which were closer to the ECOFFs to detect protein abundance differences resulting in modest MIC changes. Both EUCAST breakpoints and the selected breakpoints are shown in Supplementary Table 3.
Whole genome sequencing
DNA isolation and whole genome sequencing of all isolates was performed by Microsynth AG (Balgach, Switzerland). Extraction, lysis and isolation was performed using the Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany). Bead beating was done in 2 mL screwcap tubes containing 0.6 g 0.1 mm glass beads by a FastPrep-24™ instrument using four cycles of 45 s (MP Biomedicals, Santa Ana, United States). Subsequently, 200 µL raw extract was prepared for DNA-isolation. The isolated DNA concentration was assessed with PicoGreen measurement (Quant-iT™ PicoGreen™ dsDNA Assay Kit, Thermo Fisher Scientific, Waltham, United States). For whole genome sequencing Illumina Nextera XT libraries were prepared using the Nextera XT DNA Library preparation kit (Illumina, San Diego, United states) according to manufacturer’s instructions. Subsequently, the libraries were checked for quality and library size on a 2100 Bioanalyzer instrument using a High Sensitivity DNA Assay kit (Agilent Technologies, Santa Clara, United States). The final libraries were quantified using a Quant-iT™ PicoGreen™ ds DNA Assay Kit (Thermo Fisher Scientific, Waltham, United States) and equimolarly pooled prior to sequencing. Sequencing was performed on an Illumina NextSeq 500/550 sequencing system using a NextSeq 500/550 High Output Kit v2.5 for 300 cycles (Illumina, San Diego, United States). The produced 2 × 150 bp paired-end reads passing Illumina’s chastity filter were demultiplexed with the software bcl2fastq (version 2.19.1.403, Illumina, San Diego, United States) allowing a maximum of one mismatch per index. Quality of the sequencing reads in fastq format was checked with the software FastQC (version 0.11.7). Genomes were assembled using Unicycler v0.4 with default parameters41.
Reagents for lysis and digestion prior to LC–MS/MS
All reagents were provided by Thermo Fisher Scientific (Waltham, United States) unless otherwise specified. Dithiothreitol (DTT) and iodoacetamide (IAA) were dissolved in 50 mM ammonium bicarbonate (Sigma-Aldrich, St. Louis, United States). The digest buffer contained 50 mM triethylammonium bicarbonate buffer, 5% acetonitrile (Lab-Scan, Gliwice, Poland) and 0.5% w/w sodium deoxycholate (Sigma-Aldrich, St. Louis, United States) in water. The lysis buffer was identical to the digest buffer except sodium deoxycholate ratio was 5% w/w and DTT was added to a final concentration of 7.5 mM in the lysis buffer. TPCK treated trypsin was provided by Worthington (New Jersey, USA) and was dissolved in 50 mM formic acid, final concentration 1 μg/μL.
Sample preparation LC–MS/MS
To prepare samples for LC–MS/MS, bacterial pellets were lysed in 300 μL lysis buffer and incubated for 10 min at 80 °C and 450 rpm (Eppendorf ThermoMixer® C, Eppendorf, Hamburg, Germany). Lysates were sonicated for 2 min, 70% amplitude on a Digital Sonifier® (Branson, Danbury, USA). Subsequently, 50 μL was transferred to a new tube and 22.5 μL DTT (50 mM) was added followed by 20 min of incubation at 60 °C and 450 rpm. Samples were cooled to room temperature and alkylated with 25 μL IAA (100 mM) followed by 30 min incubation in the dark at room temperature. Subsequently, proteins were precipitated by washing twice with 900 μl acetone stored at − 20 °C (Sigma-Aldrich, St. Louis, United States). Next, 100 μL digest buffer was added and samples were sonicated for 90 s, 70% amplitude to dissolve the pellet. Ten μL trypsin solution was added and samples were incubated overnight. To stop the reaction 17 μL 5% TFA in water was added and samples were centrifuged for 10 min (21,000g, 4 °C). Supernatant was transferred to a new tube. A Pierce™ Quantitative Colorimetric Peptide Assay was used to analyze the protein concentration of all digests. All samples were vacuum dried (Savant™ SpeedVac™ Concentrator, Thermo Fisher Scientific, Waltham, United States) and stored at − 80 °C.
LC–MS/MS
Digests were diluted with 0.1% trifluoracetic acid to a final peptide concentration of 200 ng/μL. Five μL of each injected sample was analyzed using a nano-LC (Ultimate 3000RS, Thermo Fisher Scientific, Germering, Germany). After preconcentration and washing the samples on a C18 trap column (1 mm × 300 μm internal diameter (ID), Thermo Fisher Scientific), samples were loaded onto a C18 column (PepMap C18, 75 µm ID × 250 mm, 2 μm particle and 100 Å pore size, Thermo Fisher Scientific) using a linear 90-min gradient (4–38% ACN/H20; 0.1% formic acid) at a flow rate of 300 nL/min. The separation of the peptides was monitored by a UV detector (absorption at 214 nm). The nano-LC was coupled to an Orbitrap Fusion Lumos (Thermo Fisher Scientific, San Jose, CA, USA). The Orbitrap Fusion Lumos was operated in data dependent acquisition (DDA) mode. Full scan MS spectra (m/z 375–1500) in profile mode were acquired in the Orbitrap with a resolution of 120,000 after accumulation of an AGC target of 400,000. A top speed method with a maximum duty cycle of 3 s was used. In these 3 s the most intense peptide ions from the full scan in the Orbitrap were fragmented by HCD (normalized collision energy 30%) and measured in the iontrap with a AGC target of 10,000. Maximum fill times were 50 ms for the full scans and 50 ms for the MS/MS scans. Precursor ion charge state screening was enabled and only charge states from 2 to 7 were selected for fragmentation. Dynamic exclusion was activated after the first time a precursor was selected for fragmentation and excluded for a period of 60 s using a relative mass window of 10 ppm. Lock mass correction was activated to improve mass accuracy of the survey scan. After each measurement the column was rinsed with a blank to minimize carry-over. After each ten samples a quality control was measured to assess shifting retention times and data quality.
WGS data analysis
WGS data was first annotated with Prokka v1.1342. All genomes were analyzed to identify curated AMR genes using a stand-alone version of the Resistance Gene Identifier (RGI) v5.1.0 based on the Comprehensive Antibiotic Resistance Database (CARD) v3.0.543. Only perfect and strict hits were allowed. COG analysis18 was performed with WebMGA44. Statistics and visualizations were performed in R45. For E. coli and K. pneumoniae, cgMLST was performed using SeqSphere (Münster, Germany) and available core gene sets of 2513 genes for E. coli and 2358 genes for K. pneumonia. BioNumerics (Applied Maths, Sint-Martens-Latem, Belgium) was used to depict clustering of isolates by minimum spanning trees.
DDA data processing and analysis
MaxQuant 1.6.1.0 (Max Planck Institute for Chemistry, Mainz, Germany) was used to analyze the DDA data; default settings were used unless indicated otherwise. A maximum of two missed cleavages was allowed. Oxidation was set as a variable modification of methionine, carbamidomethylation as a fixed modification of cysteine, and trypsin was set as enzyme. The used protein database was assembled using the WGS data which was first annotated using Prokka v1.1342. However, as many proteins were annotated as hypothetical proteins, annotation was repeated using the protein sequences of all bacteria and plasmid entries of the NCBI RefSeq database (29th of April 2020, 138,661,652 entries). This was performed using the protein–protein basic local alignment search tool (blastp, version 2.6.046). In MaxQuant, the label free quantitation option with matching between runs was used. The quantitative values for all identified protein groups were further analyzed in Perseus 1.6.1.2 (Max Planck Institute for Chemistry, Mainz, Germany). The data were annotated, log2 transformed, and imputation was applied for missing values (in which missing values were replaced by values from a down shifted normal distribution of the intensities). Subsequently, hierarchical clustering and statistical analyses were performed to generate significance tables and protein intensity tables. To identify proteins of which the abundance was significantly correlated to resistance, a volcano plot analysis was performed based on unpaired t-tests. The cut-off for significance was based on 250 randomizations of the data set and was set at a false discovery rate of 5%. Proteins of AMR mechanisms curated in CARD were analyzed separately. These mechanisms were considered present when the encoding gene was present and at least one specific peptide of the protein was detected. Porin intensity plots were made using GraphPad Prism (GraphPad Software, San Diego, USA). OmpC variant sequences were compared using Clustal W version 2.147. Similar to the analysis of WGS data, WebMGA and BioNumerics were used for COG analysis and clustering, respectively.
Transparency declarations
The ErasmusMC is patent holder of “mass spectrometric determination of drug resistance” (PCT/NL2013/050255) which is licensed to Da Vinci Laboratory Solutions (Rotterdam, the Netherlands).
Supplementary Information
Acknowledgements
We thank Alkis Vatopoulos for providing the VIM-positive K. pneumoniae isolates and the Regional Institute of Gastroenterology and Hepatology in Cluj Napoca for providing us with the OXA-48 K. pneumoniae isolates. We thank Evosep Biosystems for allowing us to work with the Evosep One. We thank Jan Tommassen for providing us with his knowledge on porin structure and physiology. Furthermore, we thank the Erasmus MC Cancer Computational Biology Center (CCBC) for providing their IT resources and sharing their software.
Author contributions
D.E.F. and W.H.F.G. wrote the main manuscript text. N.S., C.H.W.K., L.J.M.D. and D.E.F. created the figures. D.E.F., M.T.K and L.J.M.D. performed the laboratory work. D.E.F., N.S., C.S., C.H.W.K, W.H.F.G., and L.J.M.D performed the analyses. N.S., C.S. and L.J.M.D. curated the data. T.M.L. and W.H.F.G. obtained funding and performed administrative tasks for the project. W.H.F.G., T.M.L., L.J.M.D., C.H.W.K. and A.V. supervised the project. All authors reviewed and commented on the manuscript.
Funding
This work was supported by both the Netherlands Enterprise Agency and the European Union through Horizon 2020 by means of a Eurostars grant (project number 11873).
Data availability
The genomic sequencing data of the 187 E. coli and K. pneumoniae isolates are available in the ENA repository under the primary accession number PRJEB41042 and secondary accession number ERP124768. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE48 partner repository with the dataset identifier PXD023736 for the E. coli data and PXD023739 for the K. pneumoniae data. All other data is included in this article or as “Supplemental material”.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dimard E. Foudraine and Nikolaos Strepis.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91905-w.
References
- 1.WHO. Antimicrobial resistance: Global report on surveillance 2014. https://www.who.int/drugresistance/documents/surveillancereport/en/ (2014).
- 2.Ahmed M. Acute cholangitis—An update. World J. Gastrointest. Pathophysiol. 2018;9:1–7. doi: 10.4291/wjgp.v9.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laupland KB. Incidence of bloodstream infection: A review of population-based studies. Clin. Microbiol. Infect. 2013;19:492–500. doi: 10.1111/1469-0691.12144. [DOI] [PubMed] [Google Scholar]
- 4.Cassini A, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (2017).
- 6.Stoesser N, et al. Predicting antimicrobial susceptibilities for Escherichia coli and Klebsiella pneumoniae isolates using whole genomic sequence data. J. Antimicrob. Chemother. 2013;68:2234–2244. doi: 10.1093/jac/dkt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Consortium, C.R., et al. Prediction of Susceptibility to first-line tuberculosis drugs by DNA sequencing. N. Engl. J. Med. 2018;379:1403–1415. doi: 10.1056/NEJMoa1800474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon NC, et al. Prediction of Staphylococcus aureus antimicrobial resistance by whole-genome sequencing. J. Clin. Microbiol. 2014;52:1182–1191. doi: 10.1128/JCM.03117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charretier Y, Schrenzel J. Mass spectrometry methods for predicting antibiotic resistance. Proteom. Clin. Appl. 2016;10:964–981. doi: 10.1002/prca.201600041. [DOI] [PubMed] [Google Scholar]
- 10.Wan Nur Ismah WAK, et al. Prediction of fluoroquinolone susceptibility directly from whole-genome sequence data by using liquid chromatography-tandem mass spectrometry to identify mutant genotypes. Antimicrob. Agents Chemother. 2018;62:e01814-17. doi: 10.1128/AAC.01814-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Martinez L. Extended-spectrum beta-lactamases and the permeability barrier. Clin. Microbiol. Infect. 2008;14(Suppl 1):82–89. doi: 10.1111/j.1469-0691.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 12.Hebert AS, et al. The one hour yeast proteome. Mol. Cell Proteom. 2014;13:339–347. doi: 10.1074/mcp.M113.034769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang CJ, et al. Diagnosis of beta-lactam resistance in Acinetobacter baumannii using shotgun proteomics and LC-nano-electrospray ionization ion trap mass spectrometry. Anal. Chem. 2013;85:2802–2808. doi: 10.1021/ac303326a. [DOI] [PubMed] [Google Scholar]
- 14.Trip H, et al. Simultaneous identification of multiple beta-lactamases in Acinetobacter baumannii in relation to carbapenem and ceftazidime resistance, using liquid chromatography-tandem mass spectrometry. J. Clin. Microbiol. 2015;53:1927–1930. doi: 10.1128/JCM.00620-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welker M, van Belkum A. One system for all: Is mass spectrometry a future alternative for conventional antibiotic susceptibility testing? Front. Microbiol. 2019;10:2711. doi: 10.3389/fmicb.2019.02711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foudraine DE, et al. Accurate detection of the four most prevalent carbapenemases in E. coli and K. pneumoniae by high-resolution mass spectrometry. Front. Microbiol. 2019;10:2760. doi: 10.3389/fmicb.2019.02760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Belkum A, et al. Innovative and rapid antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2020;18:299–311. doi: 10.1038/s41579-020-0327-x. [DOI] [PubMed] [Google Scholar]
- 18.Tatusov RL, Galperin MY, Natale DA, Koonin EV. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28:33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacoby GA. AmpC beta-lactamases. Clin. Microbiol. Rev. 2009;22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livermore DM. beta-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 1995;8:557–584. doi: 10.1128/CMR.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010;13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacoby GA. Beta-lactamase nomenclature. Antimicrob. Agents Chemother. 2006;50:1123–1129. doi: 10.1128/AAC.50.4.1123-1129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doi Y, Wachino JI, Arakawa Y. Aminoglycoside resistance: The emergence of acquired 16S ribosomal RNA methyltransferases. Infect. Dis. Clin. N. Am. 2016;30:523–537. doi: 10.1016/j.idc.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Putten BCL, et al. Quantifying the contribution of four resistance mechanisms to ciprofloxacin MIC in Escherichia coli: A systematic review. J. Antimicrob. Chemother. 2019;74:298–310. doi: 10.1093/jac/dky417. [DOI] [PubMed] [Google Scholar]
- 25.Babini GS, Livermore DM. Are SHV beta-lactamases universal in Klebsiella pneumoniae? Antimicrob. Agents Chemother. 2000;44:2230. doi: 10.1128/AAC.44.8.2230-2230.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassing RJ, et al. Detection of amino acid substitutions in the GyrA protein of fluoroquinolone-resistant typhoidal Salmonella isolates using high-resolution mass spectrometry. Int. J. Antimicrob. Agents. 2016;47:351–356. doi: 10.1016/j.ijantimicag.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Vergalli J, et al. Porins and small-molecule translocation across the outer membrane of Gram-negative bacteria. Nat. Rev. Microbiol. 2020;18:164–176. doi: 10.1038/s41579-019-0294-2. [DOI] [PubMed] [Google Scholar]
- 28.Chenia HY, Pillay B, Pillay D. Analysis of the mechanisms of fluoroquinolone resistance in urinary tract pathogens. J. Antimicrob. Chemother. 2006;58:1274–1278. doi: 10.1093/jac/dkl404. [DOI] [PubMed] [Google Scholar]
- 29.Choi U, Lee CR. Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front. Microbiol. 2019;10:953. doi: 10.3389/fmicb.2019.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masi M, Refregiers M, Pos KM, Pages JM. Mechanisms of envelope permeability and antibiotic influx and efflux in Gram-negative bacteria. Nat. Microbiol. 2017;2:17001. doi: 10.1038/nmicrobiol.2017.1. [DOI] [PubMed] [Google Scholar]
- 31.Correia S, Poeta P, Hebraud M, Capelo JL, Igrejas G. Mechanisms of quinolone action and resistance: Where do we stand? J. Med. Microbiol. 2017;66:551–559. doi: 10.1099/jmm.0.000475. [DOI] [PubMed] [Google Scholar]
- 32.Mori H, Kondo A, Ohshima A, Ogura T, Hiraga S. Structure and function of the F plasmid genes essential for partitioning. J. Mol. Biol. 1986;192:1–15. doi: 10.1016/0022-2836(86)90459-6. [DOI] [PubMed] [Google Scholar]
- 33.Stephens C. F plasmids are the major carriers of antibiotic resistance genes in human-associated commensal Escherichia coli. mSphere. 2020;5:e00709-20. doi: 10.1128/mSphere.00709-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sikora AE, et al. Structural and functional insights into the role of BamD and BamE within the beta-barrel assembly machinery in Neisseria gonorrhoeae. J. Biol. Chem. 2018;293:1106–1119. doi: 10.1074/jbc.RA117.000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soufi B, Krug K, Harst A, Macek B. Characterization of the E. coli proteome and its modifications during growth and ethanol stress. Front. Microbiol. 2015;6:103. doi: 10.3389/fmicb.2015.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma D, Garg A, Kumar M, Rashid F, Khan AU. Down-regulation of flagellar, fimbriae, and pili proteins in carbapenem-resistant Klebsiella pneumoniae (NDM-4) clinical isolates: A novel linkage to drug resistance. Front. Microbiol. 2019;10:2865. doi: 10.3389/fmicb.2019.02865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keasey SL, et al. Decreased antibiotic susceptibility driven by global remodeling of the Klebsiella pneumoniae proteome. Mol. Cell Proteom. 2019;18:657–668. doi: 10.1074/mcp.RA118.000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uddin MJ, Ma CJ, Kim JC, Ahn J. Proteomics-based discrimination of differentially expressed proteins in antibiotic-sensitive and antibiotic-resistant Salmonella typhimurium, Klebsiella pneumoniae, and Staphylococcus aureus. Arch. Microbiol. 2019;201:1259–1275. doi: 10.1007/s00203-019-01693-1. [DOI] [PubMed] [Google Scholar]
- 39.Cudic E, Surmann K, Panasia G, Hammer E, Hunke S. The role of the two-component systems Cpx and Arc in protein alterations upon gentamicin treatment in Escherichia coli. BMC Microbiol. 2017;17:197. doi: 10.1186/s12866-017-1100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paltansing S, et al. Increased expression levels of chromosomal AmpC beta-lactamase in clinical Escherichia coli isolates and their effect on susceptibility to extended-spectrum cephalosporins. Microb. Drug Resist. 2015;21:7–16. doi: 10.1089/mdr.2014.0108. [DOI] [PubMed] [Google Scholar]
- 41.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 43.Alcock BP, et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48:D517–D525. doi: 10.1093/nar/gkz1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu S, Zhu Z, Fu L, Niu B, Li W. WebMGA: A customizable web server for fast metagenomic sequence analysis. BMC Genom. 2011;12:444. doi: 10.1186/1471-2164-12-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.R Core Team . A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 46.National Center for Biotechnology Information (US). BLAST® Command Line Applications User Manual. https://www.ncbi.nlm.nih.gov/books/NBK279684/ (2018).
- 47.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 48.Perez-Riverol Y, et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019;47:D442–D450. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genomic sequencing data of the 187 E. coli and K. pneumoniae isolates are available in the ENA repository under the primary accession number PRJEB41042 and secondary accession number ERP124768. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE48 partner repository with the dataset identifier PXD023736 for the E. coli data and PXD023739 for the K. pneumoniae data. All other data is included in this article or as “Supplemental material”.