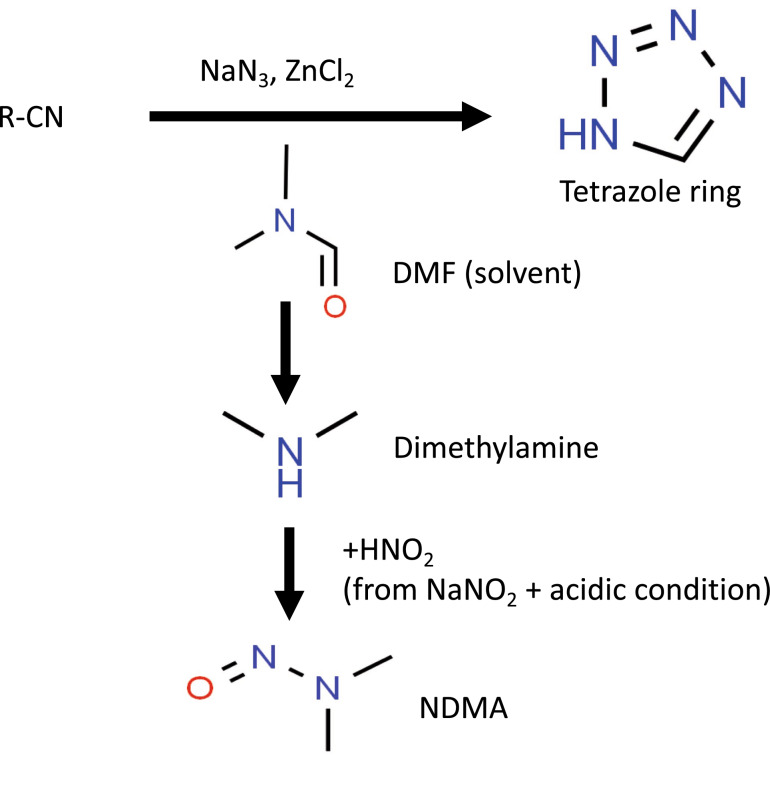
Figure 4.
Proposed chemistry of NDMA formation from DMF during sartan production. The active pharmaceutical ingredient in sartan drugs has a tetrazole ring and the conventional method to produce this ring was reported to be slow. In order to accelerate the production process, a synthesis procedure that uses the solvent, dimethylformamide (DMF) and sodium azide (in place of tributyltin azide) was introduced in 2012. During tetrazole synthesis, a small amount of dimethylamine would be formed from DMF. The synthetic process also involves the use of nitrous acid to dispose of the excess sodium azide. This nitrous acid (a nitrosating agent) which is produced from sodium nitrite under acidic conditions can react with dimethylamine to form NDMA. (R = aryl, alkyl or vinyl; DMF = dimethylformamide; NDMA = N-nitrosodimethylamine). Figure adapted from2,53–55.