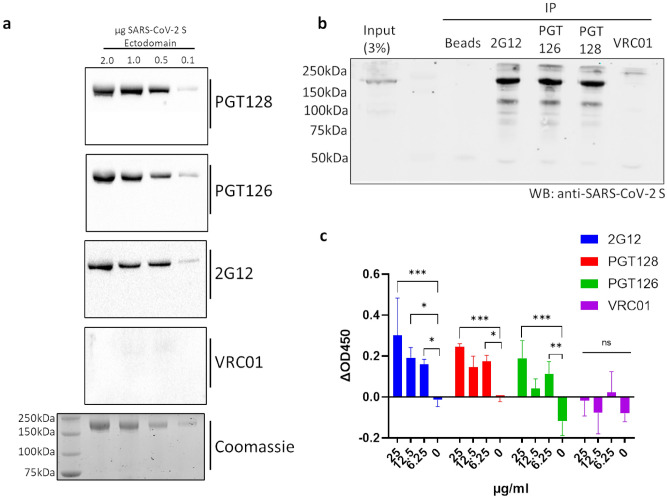
Figure 3.
SARS-CoV-2 S binding capabilities of selected cross-reactive anti-gp120 antibodies. (a) Immunoreactivity of selected anti-gp120 antibodies with the SARS-CoV-2 S ectodomain was assessed via western blot, membranes were probed with the indicated antibodies prior to detection via HRP-anti-human IgG. A Coomassie-stained gel is included as a loading control. See Fig. S4 in supplementary information for uncropped gel images. (b) Immunoprecipitation (IP) of full-length SARS-CoV-2 S by selected anti-gp120 antibodies. Lysates generated from HEK293T cells transiently expressing full-length SARS-CoV-2 S were incubated with the indicated antibodies and subjected to immunoprecipitation using protein A beads prior to Western blot (WB) analysis with a commercially available antibody targeting SARS-CoV-2 S. An immunoprecipitation condition using protein A beads alone is included as a control. Shown is a representative blot from 2 independent experiments. See Fig. S5 in supplementary information for uncropped gel images. (c) Cell-based ELISA of cell-associated full-length SARS-CoV-2 S binding by the indicated anti-gp120 antibodies. Assays were carried out on chemically fixed HEK293T cells either transfected with plasmid encoding full-length SARS-CoV-2 S, or empty plasmid (mock). The difference in signal between these conditions is presented. Experiments were done in triplicate; error bars indicate standard deviation (n = 3). Statistical significance was tested by two-way ANOVA with Dunnett post-test (P > 0.05 [ns, not significant], *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).