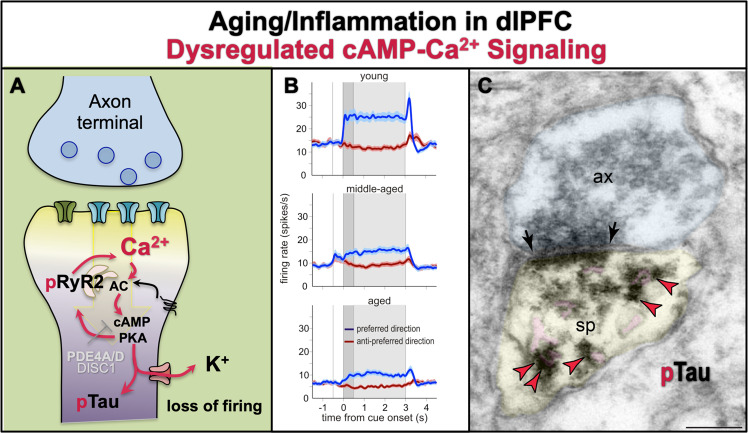
Fig. 7. The role of cAMP–calcium dysregulation in age-related pathology.
a With advancing age, there is loss of PDE4 expression from dlPFC spines [88], and increased calcium–cAMP signaling which reduces neuronal firing via excessive opening of K+ channels [162]. The increase in cytosolic calcium would be particularly detrimental when the calcium binding protein, calbindin, is lost from pyramidal cells, but not interneurons, with increased age. Gradual increases in cytosolic calcium over a long lifetime may have a number of toxic actions, including increasing tau phosphorylation (pTau) [65, 88], amyloid deposition, neuroinflammation, and ultimately, neurodegeneration. b The persistent neuronal firing of dlPFC delay cells from young adult, middle-aged, and aged monkeys performing a working memory task. The spatial cue is presented at 0 s for 0.5 s (dark gray), while the delay period ensues for the following 2.5 s (light gray shading), followed by the saccadic response to the remembered location. Strong working memory requires high levels of persistent firing for the neuron’s preferred direction (blue) compared to its nonpreferred directions (red) across the entire delay period. There was a highly significant reduction in persistent firing with advancing age, beginning in middle age when PFC cognitive deficits become evident in both monkeys and humans. Firing was restored by local inhibition of feedforward calcium–cAMP–K+ signaling. b was adapted with permission from Wang et al. [162]. c With advancing age, there is a large increase in PKA phosphorylation of tau at serine-S214, which collects on microtubules and the SER [65, 88]. This image shows pS214Tau (indicated by red arrowheads) aggregating on the SER spine apparatus (pink pseudocolor) within a dendritic spine from layer III dlPFC in an aged monkey. Ax axon terminal, sp spine, synapse indicated by black arrows; scale bar = 200 nm. Image from D. Datta.