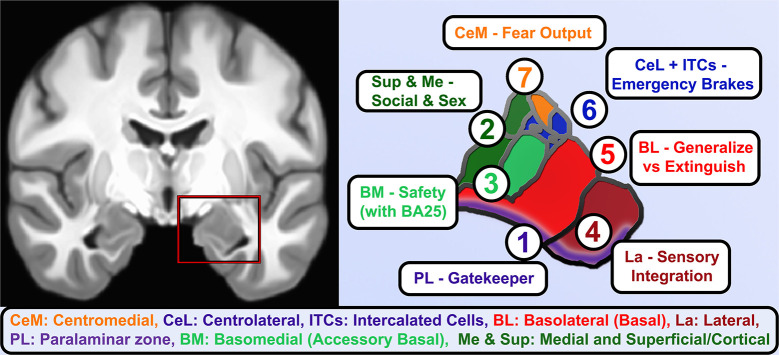
Figure 2.
Functional roles of human amygdala subnuclei. A coronal view of T1-weighted structural MRI (left) showing amygdalae resting on the anteriormost hippocampus and separated by the white matter of the alveus, and closeup of hand-segmented amygdala subnuclei (right). (1) While there is still debate about whether the paralaminar region is a true nucleus vs. the subventricular region of other nuclei, it is the main zone of from which newborn neurons migrate into the basal and lateral nuclei and houses dopamine-innervated GABAergic cells that gate activation of the basal and lateral nuclei. (2) The corticial nucleus and superficial nuclei are closely linked to the olfactory system and in vomeronasal animals coordinate responses to pheromones. As such, these and the medial nucleus of the amygdala contribute to latent drives such as recognition of conspecifics, maternal attachment, and sex-related differences and behaviors. (3) Basomedial nucleus is an early-developing nucleus that bears some functional similarities to the adjacent superficial nuclei, e.g., changing serotonin receptor expression in studies of early maternal separation, but also brings in information about safety cues from higher centers through direct innervation by infralimbic/BA25 projections. (4) The lateral nucleus gathers information about threatening cues and contexts from highly processed sensory information and contextual information from hippocampus. It is the largest nucleus in humans and most reliably enlarges in allostatic load, consistent with rodent studies showing dendritic expansion as fear generalizes. (5) The Basolateral or simply Basal nucleus similarly expands volume and dendrites after inescapable stress, but it is also home of key “extinction cells” that integrate information from other nuclei and prefrontal inputs and feeding forward inhibition to contextualize or extinguish threat responses and turn off dopamine from the VTA. (6) The intercalated cell islands (ITCs) and about half the cells in the lateral division of the central nucleus (CeL) receive feedforward inhibition from basal nuclei or are directly activated by the social bonding hormone oxytocin (CeL), to inhibit latent and previously learned fear responses. They receive heavy dopaminergic innervation and send effectors to centromedial (CeM) and a similar lateral-to-medial inhibition of the ascending arousal signaling of the cholinergic basal nucleus of the stria terminalis. About half the neurons are so-called “fear on” neurons that signal latent and previously-learned fears with some threat signals from innervation by the paraventricular thalamus. (7) CeM sends long-range projections that tonically inhibit hypothalamic and brainstem autonomic centers until fear and safety signals integrated by CeL shifts toward threat and inhibits these neurons.