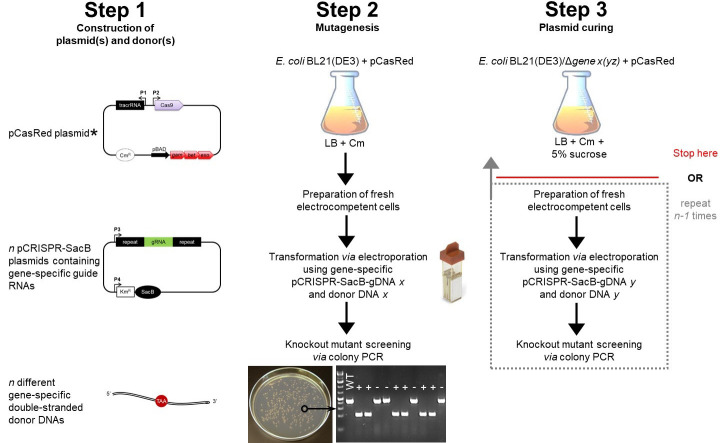
Figure 2. Schematic workflow for gene knockout in E. coli using the CRISPR/Cas9 technology.
Step 1 includes the construction of the three main components needed for this protocol (see Figure 1). The E. coli strain you want to gene-edit (e.g., E. coli BL21(DE3)) has to be transformed with the pCasRed plasmid first (note asterisk, see Step E4a.i). The E. coli + pCasRed strain is the starting point for mutagenesis (Step 2), in which fresh electrocompetent cells are prepared (Step E3) and directly used for transformation (Step E4). The next day, a colony PCR is performed to identify positive knockout mutants (Steps E5 and E6), which are subsequently inoculated in liquid LB medium containing 5% sucrose for curing of the pCRISPR-SacB-gDNA used before (Step 3). Here, the protocol can be stopped or you continue with another round of mutagenesis following only the procedures from Step 2 highlighted in the dotted box. Legend: chloramphenicol (Cm), wild type (WT), positive mutant (+), escaper (-).