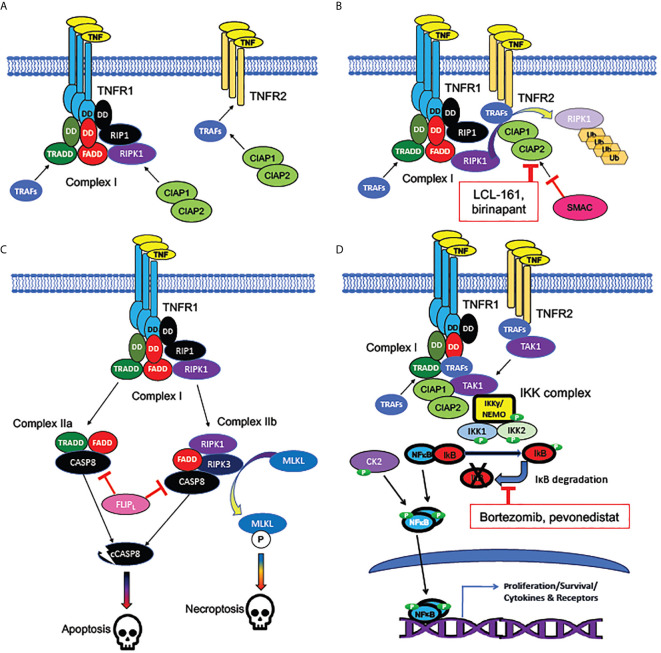
Figure 3.
Signaling pathways activated by TNF receptors. (A) Bivalent cell-death regulating Complex I formed at plasma membrane by TNFR1 but not TNFR2. TNF receptors TNFR1 (encoded by TNFSF1A) and TNFR2 (encoded by TNFSF1B) are homotrimeric receptors binding homotrimeric TNF ligand. TNFR1, unlike TNFR2, contains an intracellular “death domain” (DD), which binds homologous DDs on intracellular RIP1, FADD, and TRADD, to compose the core of Complex (I) RIP1 recruits the pro-apoptotic RIPK1 kinase, while TRADD recruits the anti-apoptotic TRAF adaptor protein family members, which are necessary for activation of NFκB and MAP kinase signaling downstream of TNFRs. TNFR2, which lacks a “death domain”, can recruit TRAFs directly, but with a lower binding affinity than Complex (I) TRAFs can recruit CIAP1 and CIAP2, anti-apoptotic proteins which form heterodimers (encoded by BIRC2 and BIRC3). (B) Mechanism of cell death inhibition by CIAPs at TNFRs. CIAPs are ubiquitin ligases, which polyubiquinate RIPK1, targeting it for degradation. CIAPs can be recruited intracellularly at TNF-bound TNFR1 or TNFR2. SMAC/DIABLO is an inhibitor of CIAPs: hence, SMAC/DIABLO mimetics (such as LCL-161 and birinapant) are pro-apoptotic. (C) Cell death pathways downstream of TNFR1. The uninhibited Complex I (with active RIPK1) promotes formation of the cytoplasmic death promoting Complexes IIa and IIb. Both complexes can activate cytoplasmic CASP8, the cleavage of which, to an active protease (cCASP8), initiates the apoptotic cascade. CASP8 activation can be inhibited by the long form of FLIP, encoded by the NFκB target gene CFLAR: a mechanism by which NFκB activation can promote cell survival. Complex IIb (containing RIPK3 as well as RIPK1) activates the kinase MLKL, which activates the necroptotic signaling cascade. (D) Activation of NFκB by TNFRs. TRAFs bound to TNFR1 or TNFR2 recruit TAK1, an essential activator of NFκB signaling. TAK1 activates the canonical NFκB pathway by recruiting the IKK complex, consisting of NEMO/IKKγ, IKK1, and IKK2. The IKK complex phosphorylates IκB family members, dissociating them from NFκB subunits and targeting them for degradation. NFκB, freed from IκB, is phosphorylated by both the IKK complex and casein kinase II (CK2), which in tandem activate NFκB subunits, which translocate to the nucleus to act as transcription factors. Bortezomib (directly) and pevonedistat (indirectly) inhibit IκB degradation. Among NFκB target genes are those encoding several cytokines and their receptors, as well as both antiapoptotic and pro-apoptotic components of the TNFR-NFκB signaling cascades.